Thought Leadership
The Unique Clinical and Economic Challenges of Rare Disease Diagnosis
By: Aseel Bin Sawad, Pharm D, MSc, MCR, MS, PhD, DBA, Global Reimbursement and Health Economics Lead at Aeglea Biotherapeutics, a company member of the Rare Disease Company Coalition
There are 7,000 to 10,000 rare diseases when looking at all rare diseases in a collective manner.1 Around 25 to 30 million Americans suffer from rare diseases which equates to 1 in every 10 individuals and 50% are children.2-4
Many rare diseases are life-threatening and progressive with substantial morbidity and early mortality.5,6 Unfortunately, only 5 to 7% of rare diseases have FDA-approved treatments, and the majority of these treatments (75%) treat only one rare disease.7
It is imperative to understand the unique challenges of the entire rare disease ecosystem to provide cost-effective and timely access to treatments for people living with rare diseases.
The Challenges
Challenges associated with rare diseases include, but are not limited to, the ability to make an accurate and timely diagnosis; overwhelming clinical burden; lack of adequate real-world data; ability to quantify the economic burden; and pricing and access.
Diagnoses that are Delayed, Undiagnosed and/or Misdiagnosed
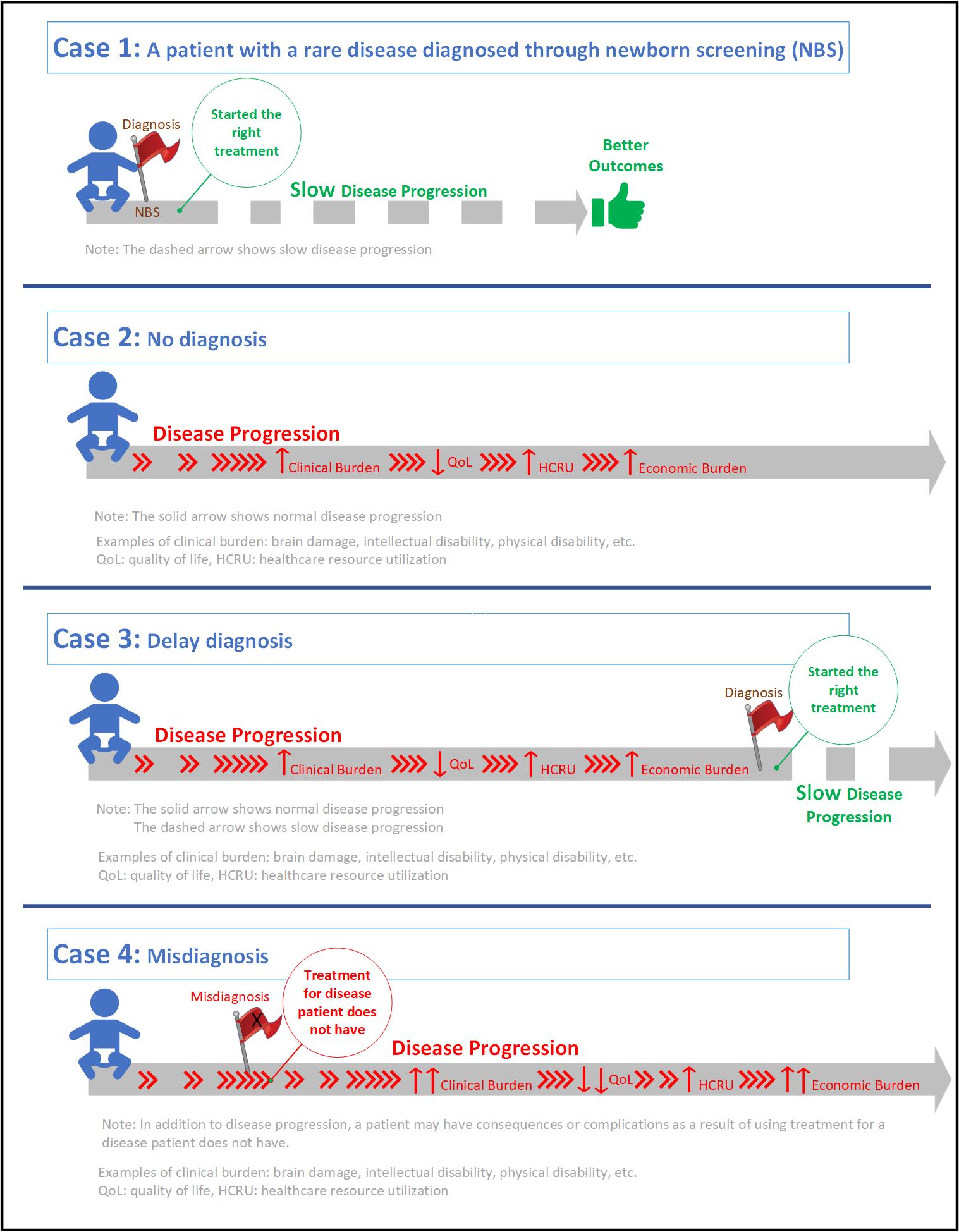
It is common for rare diseases to go undiagnosed or misdiagnosed for years, leading to serious complications such as brain damage, intellectual disability, physical disability, among others. Many rare diseases are associated with a poor quality of life due to their progressive nature, extensive utilization of healthcare resources to manage symptoms, and a substantial economic burden on the patients, caregivers and healthcare system.5,6 A recent study shows that diagnostic journeys of patients with rare diseases are lengthy and delay in diagnosis results in advanced, irreversible, and costly complications.8 Despite being born with the condition (i.e., genetic rare disease), one study reports that patients were not accurately diagnosed until an average age of 6.4 years.9
Inherited metabolic disorders are good examples of the unique challenges posed by rare diseases. While affected infants can be diagnosed through newborn screening (NBS), there are limitations. NBS is not available for most rare diseases, and for those that are included in NBS, the availability and reliability may vary from state to state. In some cases, there is lack of consistency in using appropriate analytical cut-off values for disease indicators.10,11 In addition, false negative results can occur because some plasma enzymes may take time to accumulate in newborns.12
Another challenge during the diagnosis of rare diseases is the heterogeneity of the symptoms. You can find two siblings having the same rare disease, but they have different symptoms. Similarly, many rare diseases often share similar symptomatology, leading to a misdiagnosis. Due to the extremely low prevalence of certain rare diseases, even the ‘disease experts’ may only see a handful of cases in their entire career.
Misdiagnosis can also result in a delayed diagnosis, but with extra clinical and economic burden. Patients may experience serious clinical adverse events due to medications prescribed for a disease that they do not have. In addition, further utilization of healthcare resources such as physician visits, emergency room visits, and hospitalizations, leads to extra economic burden (on the patients, caregivers and healthcare system) while patients continue the progression of their underlying, actual disease.
These examples visualize the differences between a patient who was diagnosed with a rare disease at the right time (Case 1), a patient who was not diagnosed (Case 2), a patient whose diagnosis was delayed (Case 3), and a patient who was misdiagnosed (Case 4).
We call on policymakers to make informed decisions when it comes to rare diseases, to support and expand early diagnosis programs like NBS and timely patient access to orphan drugs to enable better health outcomes.
Congress must also preserve and increase incentives to continue attracting, encouraging, and enhancing long-term investments and innovations of rare disease diagnostics and treatments. It is less expensive for the healthcare system to diagnose a patient on time versus risking complications or a massive event where a child, or others, ends up needing long-term care through state aid.
As we move into 2022, we must look at the big picture to ensure that rare disease patients live the quality of life they deserve, and receive the right diagnosis, at the right time, with the right support.
References
- Haendel M, Vasilevsky N, Unni D, Bologa C, Harris N, Rehm H, Hamosh A, Baynam G, Groza T, McMurry J, Dawkins J, Rath A, Thaxon C, Bocci G, Joachimiak MP, Kohler S, Robinson PN, Mungall C, Oprea RI. How many rare diseases are there? Nat Rev Drug Discov. 2020;19(2):77–8. https://doi. org/10.1038/d41573-019-00180-y.
- Institute of Medicine (IOM). Chapter 2. Profle of rare diseases. IOM (US) committee on accelerating rare diseases research and orphan product development. In: Field MJ, Boat TF (eds). National Academies Press (US), Washington, DC; 2010. https://doi.org/10.17226/12953.
- NCOD (National Commission on Orphan Diseases). Report of the national commission on orphan diseases. Public Health Service, U.S. Department of Health and Human Services, Rockville, MD; 1989.
- NIH NCATS. Genetics and rare diseases information center. FAQs about rare diseases. https://rarediseases.info.nih.gov/diseases/pages/31/faqs- about-rare-diseases. Accessed 06 November 2021.
- Klimova B, Storek M, Valis M, Kuca K. Global view on rare diseases: a mini review. Curr Med Chem. 2017;24:3153–8. https://doi.org/10.2174/09298 67324666170511111803.
- Ryder S, Leadley RM, Armstrong N, Westwood M, de Kock S, Butt T, Jain M, Kleijnen J. The burden, epidemiology, costs and treatment for Duchenne muscular dystrophy: an evidence review. Orphanet J Rare Dis. 2017;12:79. https://doi.org/10.1186/s13023-017-0631-3.
- NORD (National Organization for Rare Disorders). A NORD® Commissioned Report with Avalere®. Orphan drugs in the United States: An examination of patents and orphan drug exclusivity. 2021. https://rarediseases.org/wp-content/uploads/2021/03/NORD-Avalere-Report-2021_FNL-1.pdf. Accessed 07 November 2021.
- Tisdale A, Cutillo CM, Nathan R, Russo P, Laraway B, Haendel M, Nowak D, Hasche C, Chan CH, Griese E, Dawkins H. The IDeaS initiative: Pilot study to assess the impact of rare diseases on patients and healthcare systems. Orphanet J Rare Dis. 2021;16(1):1–8. https://ojrd.biomedcentral.com/track/pdf/10.1186/s13023-021-02061-3.pdf
- Bin Sawad A, Pothukuchy A, Badeaux M, Hodson V, Bubb G, Lindsley K, Uyei J, Diaz GA. The natural history, clinical outcomes and unmet needs of patients with Arginase 1 Deficiency (ARG1-D): A systematic review of case reports. Value in Health. 2021 Jun 1;24:S4.
- Cederbaum S, Therrell B, Currier R, Lapidus D, Grimm M. Newborn screening for arginase deficiency in the U.S. – Where do we need to go? Paper presented at: ACMG Annual Clinical Genetics Meeting2017.
- Therrell BL, Currier R, Lapidus D, Grimm M, Cederbaum SD. Newborn screening for hyperargininemia due to arginase 1 deficiency. Mol Genet Metab. 2017;121(4):308-313.
- Jay A, Seeterlin M, Stanley E, Grier R. Case Report of Argininemia: The Utility of the Arginine/Ornithine Ratio for Newborn Screening (NBS). JIMD Rep. 2013;9:121-124.