Press Releases
RDCC Executive Director Stacey Frisk’s Statement on the Failure to Advance the Mikaela Naylon Give Kids a Chance Act in the Senate
Washington, D.C. — “Today’s effort to advance the Mikaela Naylon Give Kids a Chance Act fell short, but the stakes remain painfully clear. New research finds that 35% of surveyed rare pediatric disease-focused biotech leaders report cancelling or delaying rare disease development programs. This is an early warning sign that the lapse of the PRV program is already slowing innovation and putting future treatment options at risk for children and families who are waiting.
“RDCC applauds the continued leadership of Sen. Markwayne Mullin, Sen. Michael Bennet, and other rare disease champions and looks forward to continuing to work with Congress on a bipartisan basis to pass the Mikaela Naylon Give Kids a Chance Act.”
Stacey Frisk, Executive Director of the Rare Disease Company Coalition
The PRV program lapsed in December 2024 – and its continued lapse will create a profound disruption for rare disease research and development. Learn more in our new report below.

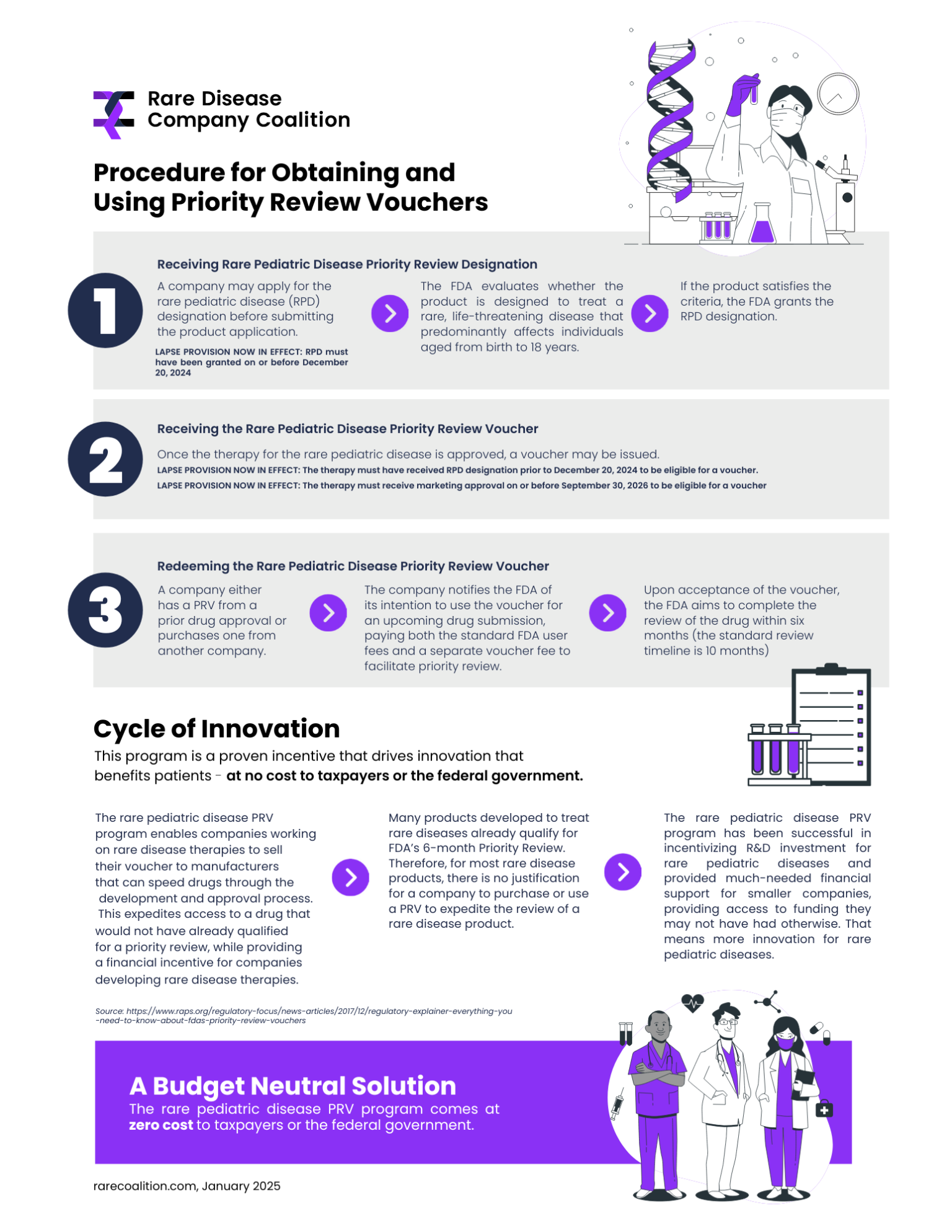
###
About the Rare Disease Company Coalition (RDCC): Founded in May 2021, the Rare Disease Company Coalition represents life science companies committed to discovering, developing and delivering rare disease treatments for the patients we serve. As an education and advocacy-focused coalition of companies, our goal is to inform policymakers of the unique challenges and promises of rare disease drug discovery, development and manufacturing for small population sizes so that critical innovation can continue and positive changes can be enacted for the rare disease community. To achieve this goal, we will use our unified voice to advocate for long-term, consistent, equitable and sustainable government policies that enable life science companies to continue to bring hope and provide access to approved treatments to people living with rare diseases. For more information, please visit rarecoalition.com.