The Rare Disease Company Coalition (RDCC) strongly supports the ORPHAN Cures Act introduced by Senators Barrasso (R-WY) and Carper (D-DE) and Representatives Joyce (R-PA-13) and Nickel (D-NC-13). The bipartisan and bicameral ORPHAN Cures Act works to encourage rare disease research and development (R&D) by addressing a critical disincentive in the Inflation Reduction Act (IRA) that impedes rare disease innovation and investment.
Currently, the IRA’s Orphan Drug Exclusion offers a price negotiation exemption for orphan drugs that treat only one orphan condition. Rare disease companies rely on this exemption as a critical incentive for R&D, but in limiting it to drugs that treat only one condition the IRA discourages rare disease companies from further exploring promising research that could lead to additional treatment options. The ORPHAN Cures Act would instead expand the Orphan Drug Exclusion to allow for the research and development of existing products that could help find treatments for the more than 95 percent of rare diseases without approved therapies.
For RDCC member companies that invest over half of their annual revenues back into research and development, improving the Orphan Drug Exclusion will have a significant impact in delivering desperately needed therapies and hope to underserved patient and caregiver communities.
Real-life impact: 1 in 5 orphan drugs are Food and Drug Administration (FDA)-approved for more than one use, and 60% of those second indications are for another rare disease. By limiting the Orphan Drug Exclusion to medicines that treat only one indication, the IRA discourages companies from pursuing development of promising therapies.
The unique needs of rare disease: The current IRA Orphan Drug Exclusion policy does not reflect the unmet need for treatments for rare diseases and the unique demands of rare disease drug development. The ORPHAN Cures Act ensures developers can continue to direct valuable resources towards rare disease research, building on the 40 years of progress enabled by the Orphan Drug Act (ODA).
The ORPHAN Cures Act strengthens the intent of the ODA: Since the enactment of the ODA in 1983, the number of orphan drugs approved by the FDA has increased from just 38 to more than 600 treatments for more than 1,000 rare diseases. While this has made a dent on the 95% of the 10,000 rare diseases that exist, continued progress requires both strengthening ODA policies and continuing to advance recognition of and support for the unique needs of rare disease R&D. The ORPHAN Cures Act achieves this by removing a disincentive for developing rare disease treatments.
The final word: The ORPHAN Cures Act addresses the concerning effects of the IRA’s Orphan Drug Exclusion and allows biopharmaceutical companies to meet the clear and urgent need for continued innovation in rare disease R&D. The RDCC thanks Senators Barrasso and Carper and Representatives Joyce and Nickel for supporting the rare disease community through this important legislation.
An Experienced DC Regulatory and Policy Counsel, Owens Brings Expertise on Navigating Rare Disease Policy Landscape
Washington, DC – November 15, 2023 – The Rare Disease Company Coalition (RDCC) recently named Graham Owens as Executive Director of the Coalition. Owens joins the RDCC at a pivotal time as the RDCC continues to advance a stronger and more stable policy environment for rare diseases. The RDCC provides a unified voice for life science companies committed to discovering, developing, and delivering rare disease treatments
“I am inspired by the great progress the Coalition has made in advancing rare disease policy and I am excited to further address the needs of RDCC companies and the patients they serve,” said Graham Owens, Executive Director of the RDCC. “As the rare disease community faces policy and regulatory uncertainty, , the RDCC plays a crucial role in advocating for investment in and support of rare disease drug development. This support ensures rare disease companies can continue to develop critical treatments for the 95% of rare diseases without an FDA-approved treatment.”
“As Chair, I am immensely excited to welcome Graham as Executive Director of the RDCC,” said Deirdre Parsons, Board Chair of the RDCC. “Graham’s professional background and energy will further advance the Coalition as the leading voice for rare disease innovators in 2024 and beyond.”
Owens most recently served as the Regulatory and Oversight Counsel for the Senate Committee on Small Business and Entrepreneurship. His experience drafting and moving legislation to ensure regulations are not “one-size-fits-all” aligns with the RDCC’s mission of promoting policies that consider the unique challenges and opportunities facing rare disease innovators today. Prior to serving in the Senate, Owens was President of Delta Agriculture where he led efforts to navigate and address key regulations and oversaw the company’s government relations portfolio, among other roles. He also previously served as the Director of Regulatory, Tax and Domestic Economic Policy for the National Association of Manufacturers (NAM). He is a graduate of the University of Virginia and the George Washington University Law School, and an active member of the District of Columbia Bar.
About the Rare Disease Company Coalition (RDCC)
Founded in May 2021, the Rare Disease Company Coalition represents life science companies committed to discovering, developing and delivering rare disease treatments for the patients we serve. As an education and advocacy-focused coalition of companies, our goal is to inform policymakers of the unique challenges and promises of rare disease drug discovery, development and manufacturing for small population sizes so that critical innovation can continue and positive changes can be enacted for the rare disease community. To achieve this goal, we will use our unified voice to advocate for long-term, consistent, equitable and sustainable government policies that enable life science companies to continue to bring hope and provide access to approved treatments to people living with rare diseases. For more information, please visit rarecoalition.com
Healthcare Capital Group Unveils White Paper Addressing Financial Risks and Policy Challenges Threatening the Future of Rare Disease Innovation
WASHINGTON, DC – (October 26, 2023) – On Wednesday, October 25, the Rare Disease Company Coalition (RDCC) hosted a congressional briefing on Capitol Hill. The briefing unveiled a new white paper, “Rare Disease Companies in the Public Markets: Challenging Performance Against a Backdrop of Policy Uncertainty.” The study analyzes the financial risks of rare disease drug development and examines the impact of policies and proposals on critical capital investment in rare disease research and development.
“Rare and non-rare are two different universes,” said Neal Masia, CEO of Health Capital Group and author of the new white paper. “There are a lot of policy risks fueling investor concerns.”
The event brought together the policy, patient advocacy, investor, and industry communities for a panel discussion on the significance of the recent decline in rare disease funding and the urgency of acting to protect and build upon the progress made over the past 40 years since the adoption of the Orphan Drug Act (ODA).
“Even the smallest shift in public policy can leave our loved ones with no hope for treatment,” said Dorothea Lantz, Director of Community Engagement at the Prader-Willi Syndrome Association. “As a rare disease community, we understand the costs and the risk of rare disease research and development.”
Panelists agreed that policy plays an integral role in incentivizing investment in rare disease R&D and that recent fluctuations have made it difficult for companies to access critical capital. The study highlights this struggle, demonstrating that the market value of rare disease companies declined by nearly 7% per year over the past five years, versus an annual decline of 1.3% for non-rare disease companies. If a more stable policy environment does not prevail, rare disease companies will continue struggling to survive.
“Investors are forward-looking. They are thinking about what the environment is going to look like 10 years from now, and they’re pretty good at connecting the dots,” said Ritu Baral, Managing Director, Senior Biotechnology Analyst at Cowen and Company. “And what can happen to turn this around? Incentives are needed to provide a buffer for current laws.”
Concerns about the policy environment have already impacted rare disease company valuations and access to capital. Rare disease companies saw nearly $10 billion less in investment available for research in 2022, stemming from decreases in venture capital investments, the IPO market, and partnership revenues. Venture capital investments in rare disease were down over 41% in 2022 and rare disease IPOs were down 81%, marking a substantial reduction of investment in rare disease companies. In a period of such financial uncertainty, seemingly small policies and proposals can have hugely consequential impacts on the ability for rare disease companies to discover, develop, and deliver potentially life-changing treatments to patients.
“Research and development is the only way that our families are going to have a chance at a normal life,” continued Lantz. “We are here to make our voices heard, not just for Prader-Willi Syndrome, but for the whole rare disease community. We hope policymakers are listening.”
Videos of Hill Briefing on October 25, 2023
A new white paper authored by Health Capital Group and sponsored by the Rare Disease Company Coalition considers the financial risks of rare disease drug development and how policy and regulatory changes can strain an already-difficult investment thesis. The study analyzed 700 publicly traded biopharmaceutical companies from mid-2018 to mid-2023 to better understand how rare disease companies and investors have been impacted by the shift in the policy environment over the past five years.
The data demonstrates that rare disease companies are struggling to survive in the current policy and funding ecosystems. Venture capital investments in rare disease were nearly halved in 2022 and rare disease IPOs were down by nearly 90%. Additionally, rare disease companies saw nearly $10 billion less in investment available for research in 2022, stemming from decreases in venture capital investments, the IPO market, and partnership revenues. Despite these challenges, rare disease companies continue to invest twice as much into research and development (R&D) as their non-rare counterparts. In a period of such financial uncertainty, seemingly small policies and proposals can have hugely consequential impacts on the ability for rare disease companies to discover, develop, and deliver potentially life-changing treatments to patients.
With 95% of over 10,000 rare diseases still lacking an FDA-approved treatment, policymakers must recognize the extraordinary unmet need for the 30 million Americans living with a rare disease. We urge policymakers to recognize the unique risks, challenges, and costs associated with rare disease drug development and support policies that promote innovation and R&D and enable access to rare disease treatments.
Read the full white paper titled, ‘Rare Disease Companies in the Public Markets: Challenging Performance Against a Backdrop of Policy Uncertainty’ here.
An overview can be found here.
By Mike Hu, cofounder of Project GUARDIAN

The benefits of early diagnosis and treatment have been well documented, with plenty of examples in cardiovascular diseases, infectious diseases, and cancer. In rare genetic diseases, early diagnosis is arguably even more critical and can bring night and day differences in outcomes, just like what my kids have shown me.
In 2011, my sons were both diagnosed with Mucopolysaccharidosis Type II (MPS II). A single nucleotide change caused a key lysosomal enzyme to be non-functional. As a result, large sugar molecules called glycosaminoglycans (GAG) accumulate in all their bodily tissues, leading to joint contractures, organ malfunction, developmental delay, and eventually premature death. Following the same enzyme replacement treatment from the age of 4 and 1, their outcomes have been very different. While my older son has very stiff joints causing difficulties even for walking, his younger brother can swim and ride a bicycle. The younger one can also put together 300-piece puzzles while the older one can’t even finish a 12-piece.
Unfortunately, early diagnosis is far from the norm in rare genetic diseases. Diagnoses are typically prompted by emerging symptoms, and often take a long time due to the rarity of such diseases (AKA the “Diagnostic Odyssey”). As the symptoms are generally signs of accumulated irreparable damage, treatment attempts at correcting the root cause are doomed to have poor efficacy: how can we expect the necrotic joints and dead neurons to be revived by the replacement enzyme that could only slow down the GAG accumulation at best? The same treatment was much more effective for my younger son who benefited from his older brother’s diagnosis and started treatment when he was pre-symptomatic, when the damage was still minimal. This, in turn, becomes a critical challenge to therapeutic development. While it’s easy to recognize the importance of enrolling patients at a time when the treatment is most efficacious, the reality is that virtually all patients are only identifiable after symptom presentation, giving the clinical trials bleak prospects from the get-go.
How can we diagnose early before symptoms emerge? How can we treat early or enroll patients when the drug candidate has the best chance to be effective? The answer lies in newborn screening (NBS). From phenylketonuria (PKU) to spinal muscular atrophy (SMA), the transformative power of early identification and effective treatment for dozens of rare genetic diseases has been demonstrated by the millions of babies whose lives have been saved. In the US, NBS is a national public health program with state infrastructure to screen for up to 63 diseases on the Recommended Universal Screening Panel (RUSP). A nomination system is in place to add conditions to the RUSP when there is sufficient evidence that newborn screening for the condition meets technical standards, is accepted by parents, and has available effective treatment. This approach is not without its own challenges, of course. The process to get a single condition through from nomination to decision takes 4-6 years on average. In the past 15 years, only 8 conditions were added to the RUSP. The lack of neonatal natural history, prospective screening data and effective treatments, and the high costs of screening are all nearly insurmountable for individual disease nominations.
GUARDIAN (Genomic Uniform-screening Against Rare Diseases In All Newborns) aims to change this. Our goal is to enable the screening of newborns using genome sequencing to identify pre-symptomatic babies and support early intervention by either using existing treatments or enrolling in clinical trials of novel treatments, all at a disease stage when treatment could be maximally efficacious. Sequencing based genomic NBS can significantly expand NBS to rare genetic diseases with a single common platform and provide synergies across conditions to decrease the cost per condition for screening and de-risk the strategy across diseases/companies supporting the platform. Investment in pilot studies of genomic NBS will support clinical trials to develop new treatments and will identify infants who are candidates for treatment once those medications/treatments are FDA approved. GUARDIAN is led by Dr. Wendy Chung who previously led the SMA NBS pilot study that serves as a prime example of a public-private collaboration, which successfully transformed a once deadly disease from no treatment and rejected by the RUSP to having multiple effective treatments and successfully being added to the RUSP. Most importantly, SMA babies undergoing timely treatment have been growing, meeting milestones, and enjoying their lives like other children. Under Dr. Chung’s leadership, GUARDIAN was launched in 2022 in New York state. With over 5000 babies already enrolled, the study is a multi-year pilot research program aimed to enroll a minimum of 100,000 newborns and screen them with a starting panel of ~250 diseases (and growing). Families with newborns who screen positive are offered information about all treatment options and clinical trials available to facilitate early treatment. Natural history and long term follow up and outcome data are being collected for all babies who screen positive. The results will be used to demonstrate the benefits of NBS for these conditions to support a RUSP nomination for routine screening.
The GUARDIAN team believes that all babies affected by severe genetic diseases deserve what the SMA babies have benefited from. We need your support! Contact us today to discuss how you can help, and together, let’s make sure all beloved babies have a healthy start.
The Rare Disease Company Coalition (RDCC) has issued a new resource outlining a set of policy recommendations that would change the nature of rare disease diagnosis in the United Sates. The recommendations find basis in the fact that the earlier patients receive an accurate diagnosis, the better their chances are of accessing effective therapies.
In issuing this new resource, the RDCC calls on policymakers and regulators to better implement existing technology and proven public health programs to ensure the healthcare system keeps pace with innovation. In modernizing the system, these policy solutions will not only ensure patients can access new therapies when needed most, but also promote the more rapid adoption of new discoveries.
The new resource also includes details on how government entities can evolve to better support early diagnosis through improved collaboration and communication. Increasing coordination and streamlining practices throughout the entire healthcare ecosystem will better equip providers and specialists to make accurate diagnosis and care for rare disease patients.
A Setback in Investment for Continued Research & Development for the Rare Disease Community
Posted June 30, 2023
CMS today released revised guidance on the Inflation Reduction Act Medicare Drug Price Negotiation Program, clarifying that a drug with more than one designation for a rare disease or condition will not qualify for the Orphan Drug Exclusion, even if the drug has not been approved for any additional indications. Unfortunately, by making orphan products with a second designation eligible for drug price negotiation, this provision will disincentivize further investment in rare disease research and development.
Why it’s important: This policy will impact the rare disease drug development pipeline for decades to come, increasing health disparities for the 30 million Americans living with rare, debilitating diseases, 50% of which affect children.
Undermining the bipartisan Orphan Drug Act (ODA): The ODA was enacted to incentivize the development of drugs for rare diseases and has demonstrated overwhelming evidence of success.
The final word: The RDCC is disappointed in CMS’s revised guidance and remains committed to working with stakeholders in the Administration and Congress to find ways to mitigate the impact of this policy on Americans living with rare diseases.
By Giacomo Chiesi, Head of Chiesi Global Rare Diseases
As a certified Benefit Corporation (B Corp) serving the needs of rare disease patients, we believe we have a societal obligation to address policies impacting the estimated 350 million people living with a rare disease. The unmet need is overwhelming, as less than 5-10% of known rare diseases have a treatment. A recent call from leaders in the United States Congress to ban the use of the Quality Adjusted Life Year (QALY) metric in all federal programs could go a long way toward correcting profound societal inequities impacting rare disease patients and caregivers – and may provide a financial benefit as well.
QALY-based assessments imply that the value of an intervention is proportional to the beneficiary’s capacity to benefit, which therefore favors those with “more treatable” conditions and those with greater potential for health, in terms of functioning and/or longevity. We, alongside our colleagues at the Rare Disease Company Coalition, argue that while QALY can be a useful part of assessing the economic value of healthcare treatments and outcomes for high prevalence diseases, use of QALY for orphan drugs is a prime example of how blunt use of traditional health policy can discriminate against rare disease patients.
The traditional QALY measure focuses on gain in lifespan but does not reflect health gains reported by patients with rare diseases that have few if any treatment options available; nor does it incorporate what patients value in a treatment such as less frequent dosing or reduction in infusion schedules. Fortunately, the use of QALY to assess the value of cell and gene therapy for the treatment of rare diseases has been, in few cases, positive as it drives HTAs/payors to arrange value-base or managed access agreements to manage the high upfront cost of these therapies, enabling access.
As health care costs often begin in childhood for about half of all rare diseases, this can perpetuate a misconception among payers that rare disease treatments are too costly. This misconception further discriminates against a vulnerable population and overlooks that the cost of therapeutics for rare diseases is small compared to the average annual economic burden shouldered by patients and their families.
That misconception exists because some issues disproportionately impact the rare disease community, including process of care, indirect costs such as productivity loss, long term or short term disabilities, home changes, and traveling and accommodations for medical visits.
In 2020, Chiesi Global Rare Diseases commissioned a health economics study to measure the societal value of pharmaceutical treatments for rare diseases and inform policymakers about the unmet needs of the rare disease community in the United States. We estimated that the overall burden of rare diseases in the U.S. is $7.2 trillion to $8.6 trillion per year. That is approximately ten times higher than high-prevalence diseases on a per patient per year (PPPY) basis. A lack of treatment for a rare disease was associated with a 21.2 percent increase in total costs.
While most rare disease direct costs are covered by insurers, we found that an important part of indirect costs remain on the shoulders of patients and families. People living with a rare disease often depend on a family caregiver, which impacts that individual’s health, quality of life and economic stability as well. When no treatments were available, our study showed that the range for productivity loss per year was approximately $33,000 to $61,000 for patients and $25,000 to $61,000 for caregivers, compared with approximately $3,000 to $22,000 for patients and $4,000 to $5,000 for caregivers when treatments were available.
These findings highlight that providing access to rare disease treatments generates substantial value for society because it potentially lowers the associated economic burden on patients and caregivers. But assessments like QALY do not consider the indirect costs or non-health-related benefits such as the ability for caregivers to work and improved productivity at work. Also omitted in QALY assessments are patient and caregiver reported outcomes, such as value of hope and value of knowing. Both can provide quantitative and relevant information regarding the impact on daily life.
Declaring access to rare disease therapies a public health priority will likely lead to further investment in treatments. A public health priority designation can lead to restored incentives and further rare disease drug development and would support ongoing Congressional calls for a coordinated approach at the U.S. Food and Drug Administration (FDA) to provide regulatory reliability and expertise in its rare disease product reviews.
Public policy should increase caregiver resources and provide relief for families affected by rare diseases, as they bear high indirect or non-reimbursed expenses. Congress can and should do more by investing in the care economy, recognizing that rare disease family caregivers are an important segment of the infrastructure equation. We often hear mixed messages from government regarding access, drug pricing and innovation that risk perpetuating existing health disparities. We need to recognize that working together for the unified purpose of delivering treatments for rare disease patients is a societal imperative. The dignity and insight of the rare disease patient and caregiver should inform our progress.
Learn more about improving patient access to life-changing, FDA-approved rare disease therapies in this new Rare Disease Congressional Coalition policy paper.
The Rare Disease Company coalition (RDCC) is pleased to share this new resource for federal and state policymakers and regulators to help inform legislative actions that can bolster patient access to life-changing, FDA-approved rare disease therapies.
Today, over 95% of rare diseases lack an FDA-approved treatment, and while the FDA continues to add to the number approved therapies, treatments are only as valuable as they are accessible.
Once a rare disease treatment is approved by the FDA, patients seeking to benefit from the approved therapy are often faced with challenges to access due to complex interstate credentialing, limited coverage, delays in review, inaccurate value frameworks, and insufficient medical necessity determinations.
In this new resource, the RDCC outlines six areas for improvement with specific policy solutions that will improve outcomes for the rare disease community.
The RDCC sent a letter to Congress opposing recent policy proposals that undermine the intent of the accelerated approval pathway, and would limit patient access to life-saving treatments for rare disease patients. Any future policy reforms to accelerated approval should protect patient access, build upon Congress’ recent steps to boost FDA oversight of accelerated approval products, leverage and enhance its existing authorities, and protect the integrity of this critical pathway.
The RDCC calls on Congress to reject these proposals by:
For 30 years, the accelerated approval pathway has served as a critical lifeline for rare disease patients, leading to significant advances in the treatment of serious, life-threatening diseases. Congress and the FDA have regularly reaffirmed that medical products approved through the accelerated approval pathway meet the nation’s stringent standards for safety and effectiveness. The policy proposals currently being brought forward would upend past progress and prevent future discovery.
Click here to download and read the letter sent to Members of key Congressional committees.

The RDCC has shared a letter with Minnesota State legislators in support of four new bills that have the potential to better the lives of those living with a rare disease. The work being done in Minnesota shows the important impact state-level policy can have on rare disease drug development, both in an individual state and beyond.
These four bills will have an immediate and lasting impact on Minnesotans facing a rare disease. At the same time, we urge the legislature to reject a new proposal to create a Prescription Drug Affordability Board (PDAB) in Minnesota. A PDAB would hinder the development of, and access to, existing and future rare disease therapies and would significantly undermine the meaningful work being done to bring more treatments to rare disease patients.
Click here to download and read the letter sent to the Members of the Minnesota Legislature.
The Rare Disease Company Coalition (RDCC) supports Cameron’s Law and the Rare Disease Clinical Trial Pandemic Disruption Act (now called Leo’s Law), two important bills reintroduced this week by Representatives Josh Gottheimer and Don Bacon in honor of Rare Disease Day. These legislative proposals would help to close the innovation gap for rare disease research by encouraging more upfront investment in R&D and by promoting flexible clinical trials that encourage the pursuit of promising treatment options for rare disease patients.
40 years ago, the Orphan Drug Act (ODA) recognized the critical need to provide incentives for rare disease research. The RDCC has called on policymakers to preserve policies that will continue to attract and maintain long-term investment in the development of advanced treatments and diagnostics for rare diseases, and the reintroduction of both these bills is a critical step toward this goal.
Specifically, Cameron’s Law would restore the tax credit for clinical testing expenses from 25% back to the original 50%. Increasing the tax credit to its original parameters would make it more economically feasible for drug companies to develop rare disease treatments and provide needed certainty for continued investment.
The ODA also established the importance of exclusivity for drugs with orphan designation as a critical incentive for rare disease drug development. The Rare Disease Clinical Trial Pandemic Disruption Act (now called Leo’s Law) would extend exclusivity for rare disease clinical trials stalled during the pandemic and ensure promising cures get to the patients who need them.
The RDCC thanks Representatives Josh Gottheimer and Don Bacon for their bipartisan leadership that will directly benefit the lives of the one in 10 people living with a rare disease—half of whom are children. As a coalition of life science companies committed to discovering, developing, and delivering rare disease treatments for the patients we serve, the RDCC is eager to further engage and use our #OneRareVoice to move these bills forward in the 118th Congress.
Amanda Malakoff, Executive Director of the Rare Disease Company Coalition, was invited to speak during FDA’s Rare Disease Day 2023 virtual public meeting on February 27th. Please see below for a transcript of her remarks.

My name is Amanda Malakoff, and I serve as Executive Director for the Rare Disease Company Coalition, or the RDCC. To provide a brief background on us, we launched two years ago to represent the unique policy needs of 20+ rare disease companies working to develop and deliver rare disease treatments to patients. Collectively, our 21 members invest over $12 billion annually in research and development with over 200 new treatments in their pipelines.
We want to thank the FDA for making rare disease drug development a growing priority at the agency. The RDCC applauds relevant programs passed in the user fee authorization bill such as the Rare Disease Endpoint Advancement Pilot Program, increased funding for FDA, ARPA-H, and NCATS for rare disease research, policies aimed at improving clinical trial diversity, and more. We also are thrilled to see CDER’s recent launch of the Accelerating Rare disease Cures program.
Despite recent cutting-edge medical breakthroughs in rare disease biotech, and these supportive regulatory effort, many challenges still remain in getting treatments to the rare disease patients who need them in an urgent manner.
For example, rare disease company sponsors cite inconsistent feedback across review divisions as an ongoing barrier to meeting regulatory timelines. As FDA gears up to hire many new reviewers in PDUFA VII (7), and initiates a review of drug approval trends across review divisions, we recommend that the agency streamline the approach to training new reviewers on rare disease drug development. In addition to internal consults, external consultation with patients, patient groups, disease-specific experts, and small population study experts will help the agency make well-informed decisions and avoid roadblocks in providing patients with timely access to new treatment options.
We also encourage our agency partners at the FDA to use the flexibility granted by Congress with respect to standards for conducting rare disease trials in a consistent manner. One critical tool for rare disease drug sponsors is the accelerated approval pathway, which can offer hope to patients by providing life-changing, and sometimes life-saving, treatments years earlier. Although a significant percentage of the agency’s accelerated approvals have been for oncology, we believe this can be a critical pathway for rare diseases as well and would like to see the agency provide the appropriate support for rare disease drug sponsors who opt to use this regulatory pathway. As many rare diseases are progressive in nature, and around half affect children, time is crucial. The RDCC looks forward to serving as a constructive partner on rare disease drug development issues with the agency and key stakeholders in the rare disease community. Thank you for the chance to engage in today’s important conversation.
The Rare Disease Company Coalition today issued the following statement from Executive Director Amanda Malakoff in response to the three payment models released by Health and Human Services for testing by the Centers for Medicare & Medicaid Services. The proposal, which aims to reduce Medicare spending for some accelerated approval drugs, undermines the intent of the pathway and mischaracterizes the extent of sponsors’ clinical research. Any attempt at indication-based pricing by the US government would have enormous impacts on rare disease patients, as well as on the companies investing in critical research.
Amanda Malakoff, Executive Director, Rare Disease Company Coalition
The FDA’s accelerated approval pathway recognizes that a “one-size-fits-all” traditional model for the approval of new medicines cannot always work – especially for rare diseases. This well-established, proven, scientifically rigorous path forward enables rare disease companies to make promising new and improved treatments available to patients at the earliest possible point in time for use in life-threatening and serious rare diseases.
Passed 40 years ago, it may be time for ODA 2.0
By: Steve Usdin, Washington Editor, BioCentury
As the Orphan Drug Act slides into middle age, patient advocates and biopharma companies are applauding its accomplishments, lamenting legislative setbacks that have limited its utility, and developing proposals for expanding its reach.
In the 40 years since the ODA was signed into law, FDA has approved more than 600 drugs for orphan conditions, up from 10 in the prior decade. The success has been tangible, but new policies will be needed to increase the pace of drug development if society is to come close to meeting the needs of the millions of patients living with thousands of rare conditions that have no treatments.
By: Sheila Frame, President, Americas at Amryt Pharma and serves as a Board Member of the Rare Disease Company Coalition. She has spent more than 20 years in the biopharmaceutical industry, including in commercial leadership roles at Novartis and Bristol Myers Squibb.
Originally Published at RealClearScience.com (January 18, 2023)
The United States Congress is officially a house divided with the two chambers controlled by rival parties. The split will significantly affect either party’s ability to pass significant legislation through Congress, possibly resulting in two years of partisan deadlock that may remain unresolved until the next election cycle in 2024. Political polarization has become our new normal. Yet, I believe there are some areas where Americans can and do stand united. One of those areas: supporting health care for our kids.
In the US today, approximately 30 million people live with a rare disease, about half of whom are children. While many of these diseases are inherited at birth, they are also chronic, can worsen over time, and are often life-threatening. As the 118th Congress begins in January and works to identify areas of bipartisan compromise – accelerating diagnosis, advancing cures and supporting access to patient care for these 15 million kids can be a bipartisan victory.
It is important to recognize achievements from the 117th Congress that will disproportionately benefit kids with rare disease. In particular, the reauthorization of the Prescription Drug User Fee Act included the establishment of a Rare Disease Endpoint Advancement (RDEA) Pilot Program. This new program seeks to advance rare disease drug development programs by providing new ways for clinical trial sponsors to collaborate with the U.S. Food & Drug Administration (FDA). This will support identification of new endpoints to measure a medicine’s effectiveness, hopefully leading to more effective rare disease therapies while reaching patients sooner. This program is a noteworthy example of the progress that can be accomplished when policymakers and the innovative biopharmaceutical community collaborate with patients’ best interests in mind. Yet, with over 90% of rare diseases without an FDA-treatment, there is so much work left to do.
Continue reading at RealClearScience.com.
By Dr. Brian Roberts, Chief Medical Officer, Rezolute Bio, a member company of the Rare Disease Company Coalition
We are in a pivotal era of rare disease innovation that originated 40 years ago with the passage of the Orphan Drug Act (ODA). As a practicing endocrinologist, researcher and drug developer with considerable experience in the rare disease area, it is humbling for me to witness the many research institutes, biotech companies, policy organizations and patient advocacy groups that are unwavering in their commitment to those impacted by rare illnesses. I stand with them in their commitment to creating novel, life-changing treatments for rare and ultra-rare diseases and helping to increase access of these therapies to patients living with these conditions.
While impressive progress has been made in the last 40 years, over 10,000 rare diseases still impact an estimated 25-30 million Americans, and at least 95% of them still lack an FDA-approved therapy. More important than the numbers are the people these diseases impact daily. Rare diseases inflict a tremendous burden on patients, their families and caregivers, often making the simplest daily tasks incredibly challenging. Thanks to the ODA and organizations like the Rare Disease Company Coalition (RDCC) that advocate for changes in health policy and incentives for significant scientific advancements, important discoveries continue to be made that positively impact patients and bring new therapies for rare disease.
A disease like congenital hyperinsulinism (HI), a serious, rare pediatric disease, affecting 1:25,000 – 50,000 children born in the U.S., is just one example of a rare disease with a high unmet need for new therapies. HI presents most commonly in the first month of life, causing dangerously low blood glucose and placing developing brains at significant risk for irreversible damage. There are no regulatory-approved medical therapies for all forms of HI. Currently available therapies are repurposed drugs that were not developed for HI or a pediatric population, have serious side effects, and are inadequate in controlling hypoglycemia for a majority of HI patients. When currently available medical therapies fail, families must choose between intensive medical/feeding regimens or a near-total pancreatectomy. New potential therapies for rare conditions like HI are now in development and are the direct result of the ODA. More policies and regulations that encourage research and development of treatments for the rare disease community are still very much needed.
Working in the rare disease space and being able to develop meaningful drug therapies is incredibly gratifying. My colleagues and I are motivated by the possibility of helping improve the lives of very specific groups of patients by providing better therapies to patients and families, who have suboptimal treatment options. That’s a great feeling. The orphan drug and rare disease revolution may have its origins in the ODA, but it’s driven by the passion and innovative thinking of physicians, scientists and the broader biotech community who are pioneering breakthrough innovations in small, entrepreneurial companies. At Rezolute, we are one such company, committed to developing bold medicines that transform the lives of patients. We are advancing RZ358 as a novel therapy for those living with HI thanks to the dedication of our team members, the close partnerships we have formed within the rare disease community and organizations such as RDCC.
While the ODA was the original impetus in launching the meteoric rise of orphan drug development, a renewed sense of urgency is now underway. Rare disease biotech companies need continued substantial incentives to support continued research and development of orphan drugs for conditions like HI, and so many more that affect millions globally. Like many biotech companies, our ability to bring forward new therapeutics is mainly dependent on successful clinical trials. While Rezolute was able to successfully complete our recent pediatric rare disease trial, in spite of challenging circumstances during the pandemic, many clinical trials were significantly impacted or even halted. Trials became even more challenging to complete, given the greater health concerns of rare disease patients and numerous logistical roadblocks. Actions to extend exclusivity for orphan drugs, whose development was disrupted during the pandemic, is just one way to incentivize companies to complete these trials and bring new treatments and cures to patients battling rare diseases.
I’m hopeful for the future of rare and orphan drug disease development and call on Congress for its full support of the ODA so the biotech community can continue working to transform the lives of patients and their families.
Learn more about the Orphan Drug Act and the RDCC’s commitment to furthering innovation for a community that long deserves life-changing treatments.
RDCC member companies are commemorating the 40th anniversary of the Orphan Drug Act (ODA) by sharing how its policies have enabled their therapeutics to reach rare disease patients around the world who have had limited or no treatment options. For 40 years, the ODA has empowered biotech companies to invest in rare disease research and innovation that would otherwise be impractical or impossible to pursue.
Read how biotech companies have relied on incentives established by the ODA, like the Orphan Drug Tax Credit, to deliver new treatments for patients living with rare diseases like hyperoxaluria type 1 and acute hepatic porphyria, facioscapulohumeral muscular dystrophy, Batten disease, enzyme deficiency diseases, tumor-induced osteomalacia (TIO), X-linked hypophosphatemia (XLH), rare blood disorders including pediatric PK deficiency, thalassemia and sickle cell disease, and more. With 95% of rare diseases still lacking a treatment option today, policymakers should renew their commitment to protecting and building on the ODA to ensure continued innovation and investment.
Pushkal Garg, MD, Chief Medical Officer and EVP, Development & Medical Affairs of Alnylam Pharmaceuticals
“Alnylam has obtained 5 drug approvals in the past 5 years, of which 4 were for medicines which have been granted an Orphan Drug Designation. We believe the speed and agility with which we were able to bring these transformative medicines to patients who are living with some of the world’s rarest diseases, such as primary hyperoxaluria type 1 and acute hepatic porphyria, would not have been possible without the provisions of the Orphan Drug Act. The provisions of the Act, importantly those that offer financial incentives and marketing exclusivity are vital for innovative Companies like Alnylam who have made sizeable investments in rare disease drug development, thereby bringing novel therapeutics and hope to patients around the world who have had limited or no treatment options, while also allowing us to continue to invest in our R&D engine to fuel the discovery and development of future breakthroughs in medicine.”

Giacomo Chiesi, Head of Chiesi Global Rare Diseases
“The Orphan Drug Act created a framework whereby industry can focus on long-term investments in R&D, but there is much work to be done. Policymakers must understand that rare disease patients and families have unique needs. Working together for the unified purpose of delivering treatments for rare disease patients is a public health imperative.”

Santiago Arroyo, M.D., Ph.D. Chief Medical Officer, Fulcrum Therapeutics
“Time is precious for patients and families affected by rare diseases. Fulcrum is proud to have launched the first Phase 3 clinical trial for facioscapulohumeral muscular dystrophy (FSHD) – a devastating, progressive neuromuscular disease for which there are no approved treatments – less than three years after becoming a public company. The incentives provided by the Orphan Drug Tax Credit are essential for enabling young companies like ours to rapidly research and develop promising new therapies for the communities that are waiting.”

Nevan Charles Elam, JD, Chief Executive Officer & Founder, Rezolute Bio
“When you look beyond rare diseases that are currently being treated or have the potential for new therapies, you see millions of people who now have hope thanks to the ODA. While the disease may be rare, the people are not, and without the ODA, the incentive to research and develop new therapies for patients in need would simply not be there.”

Jean-Jacques Bienaime, Chairman and Chief Executive Officer at BioMarin
“The Orphan Drug Act (ODA) makes it possible for us to invest in the research and development necessary to address the unmet needs of rare disease patients. Since the passage of the ODA, all of BioMarin’s FDA-approved therapies have received orphan designation. As such, BioMarin has been able to transform the lives of children across the globe who have rare diseases such as CLN2 (a form of Batten disease) and enzyme deficiency diseases, among others.” By protecting the ODA, we are protecting the future of innovation and sustaining hope for children and adults alike with rare diseases.”

Camille Bedrosian, Chief Medical Officer, Ultragenyx
“The incentive provided by the Orphan Drug Tax Credit allowed Ultragenyx to study Crysvita® for tumor-induced osteomalacia (TIO), an ultra-rare disease that most companies would never have studied, after Ultragenyx previously obtained initial FDA approval of Crysvita® for the treatment of X-linked hypophosphatemia (XLH). We support the continued existence of the Orphan Drug Tax Credit as a critical incentive for rare disease companies.”

Del Lebel, Head, U.S. Government Affairs and Policy, Alexion, AstraZeneca Rare Disease
“The Orphan Drug Act spurred tremendous progress in the development of rare diseases treatments not only in the United States, but worldwide. The law has served as a catalyst for rare disease innovation and regulatory approval pathways in many other countries. Now is the time to build on that progress and promote sound policies that will improve patients’ lives.”

Ernesto Aycardi, Chief Development Officer and Head of Global Drug Development, Kyowa Kirin
“Kyowa Kirin is focused on advancing science and providing medicines for patients with unmet needs, and we consider Orphan Diseases an opportunity to accomplish our goals of serving the neediest. The ODA incentive programs have been crucial to our industry’s work – and we celebrate the profound impact they have had for patients living with rare diseases. Given the number of patients still in search of effective treatment options, we need collective action and appropriate programs to continue making a difference and foster innovation.”

Brian Goff, Chief Executive Officer at Agios Pharmaceuticals
On the 40th anniversary of the Orphan Drug Act, we celebrate the progress that has been made for hundreds of rare disease communities who previously had no treatment options. Since the legislation’s enactment in 1983, the number of FDA-approved medicines has grown from 38 to more than 650 – and that’s no coincidence. We also recognize that more progress is needed for the 95% of rare diseases that still lack treatment. Upholding and strengthening the provisions of the Orphan Drug Act fuel ongoing innovation on behalf of underserved communities, such as Agios’ clinical programs in rare blood disorders including pediatric PK deficiency, thalassemia and sickle cell disease.”

Jeffrey M. Dayno, M.D., Interim Chief Executive Officer, Harmony Biosciences
“The Orphan Drug Act catalyzed new hope and possibilities for the millions of people living with a rare disease who had not yet had their unmet medical needs addressed by an approved new treatment option. Thanks to the tireless efforts of patients and their advocates, we can now build upon 40 years of industry rare disease research, innovation, and therapeutic breakthroughs. Harmony Biosciences is privileged to continue this mission as we strive to bring hope to people living with rare neurological diseases – ensuring that patients remain at the heart of everything we do.”

Steven Pfanstiel, Chief Financial Officer, Marinus Pharmaceuticals
“As a company committed to the development of innovative treatments for patients with rare forms of epilepsy, the Orphan Drug Act (ODA) helped enable us to deliver a first-of-its-kind treatment option to the CDKL5 deficiency disorder community for an indication with no prior approved therapies. With four additional clinical programs underway or expected to begin in 2023 utilizing the same compound, the ODA is critical to ensuring we can continue to drive innovation for our underserved patient communities in need of new treatment options.”

Chris Morabito, M.D., Chief Medical Officer, Astria Therapeutics
“Astria’s mission to provide life-changing therapies for people affected by rare diseases is directly aligned with the goals of the Orphan Drug Act and the Orphan Drug Tax Credit (ODTC). The ODTC enables companies like ours to bring important medicines to patients more efficiently, and we are supportive of the preservation of this critical incentive for the drug development process.”

2023 marks the 40th anniversary of the Orphan Drug Act (ODA) and kicks off a year of renewed hope and promise for developing rare disease medicines.
WASHINGTON, DC – January 3, 2023 – As 2023 marks the 40th anniversary of the Orphan Drug Act (ODA), the Rare Disease Company Coalition (RDCC) recognizes the advances made over the past four decades and calls on Congress to renew its support for policies that promote investment and innovation in rare disease treatments.
The ODA has spurred biopharmaceutical companies to pursue treatments that would otherwise be financially unfeasible. ODA policies reduce the risk associated with rare disease research and development (R&D) and incentivize companies to discover new treatments. Since its enactment in 1983, the number of orphan drugs approved by the Food and Drug Administration (FDA) has increased by 1,576% – from just 38 to more than 600 treatments for more than 1,000 rare diseases. Today, RDCC members collectively have over 200 promising rare disease treatments currently in development.
As we recognize the ODA’s role in incentivizing biomedical innovation, we also acknowledge that the vast majority of rare disease patients still lack any FDA-approved treatment options, necessitating urgent policy considerations to bring hope to the 1 in 10 Americans suffering from a rare disease.
“Over the past 40 years, the ODA has established a strong foundation for the biotech community to invest in and develop new medicines for rare diseases,” says Amanda Malakoff, Executive Director, Rare Disease Company Coalition. “We need to build on that strong foundation so we can ensure continued innovation for the 95% of rare diseases that currently have no approved treatments.”
Researchers and developers rely on steady and supportive policy and regulatory environments to reduce the risk of investing in new treatments for small patient populations. While the ODA has supported a stable environment for the past 40 years, recent policy changes threaten ODA-established incentives like the Orphan Drug Tax Credit. New one-size-fits-all policy proposals fail to fully address the nuances of rare disease research and development and would drastically undermine this progress.
“When you look beyond rare diseases that are currently being treated or have the potential for new therapies, you see millions of people who now have hope thanks to the ODA,” said Nevan Charles Elam, JD, Chief Executive Officer & Founder, Rezolute Bio, a member of the RDCC. “While the disease may be rare, the people are not, and without the ODA, the incentive to research and develop new therapies for patients in need would simply not be there.”
Continued progress requires policymakers be active and intentional about enacting and protecting policies that build on 40 years of the ODA. The RDCC calls on policymakers to:
“In recognizing the 40th anniversary of the ODA, it is more important than ever for policymakers, patients, patient advocacy groups, providers, researchers, and manufacturers to come together and reaffirm their commitment to the rare disease community by showing steadfast support for upholding and strengthening the ODA,” Malakoff concludes.
To learn more about the Orphan Drug Act and policy priorities from the Rare Disease Company Coalition, please visit www.rarecoalition.com
About the Rare Disease Company Coalition (RDCC)
Founded in May 2021, the Rare Disease Company Coalition represents life science companies committed to discovering, developing and delivering rare disease treatments for the patients we serve. As an education and advocacy-focused coalition of companies, our goal is to inform policymakers of the unique challenges and promises of rare disease drug discovery, development and manufacturing for small population sizes so that critical innovation can continue and positive changes can be enacted for the rare disease community. To achieve this goal, we will use our unified voice to advocate for long-term, consistent, equitable and sustainable government policies that enable life science companies to continue to bring hope and provide access to approved treatments to people living with rare diseases. For more information, please visit rarecoalition.com
Executive Director Amanda Malakoff and Lisa Feng, the Senior Director of Policy at Alexion, AstraZeneca Rare Disease (an RDCC member company) joined the Vital Health Podcast with Duane Schulthess for a discussion on the accelerated approval pathway for rare disease treatment development.
Recent proposed federal and state policies to restrict coverage and access to biomedical innovations approved through the FDA’s accelerated approval pathway would harm patient access to rare disease treatments. Vital Transformation completed a comprehensive impact assessment of these proposed changes and what they could mean for patients currently lacking treatments and future innovations. According to this research, up to 66% of accelerated approval treatments could be at high-risk of not coming to market or being developed at all if the FDA’s accelerated approval pathway were negatively impacted.
Listen below for the full discussion and click here for more information on why protection of the accelerated approval pathway for rare disease treatment is urgently needed.
We appreciate the opportunity to provide comments on EEOC’s FY 2022-2026 Draft Strategic Plan and look forward to working together to end the discriminatory practices of employer-sponsored health plans, ensuring that rare disease patients have access to the treatments and therapies they desperately need.
It is vital that EEOC exert its proper authority within its jurisdiction to prevent employers from intentionally or unintentionally discriminating against rare disease patients based on their disabilities and, in so doing, preventing those patients from accessing the transformative treatments that have become available through decades of healthcare innovation.
When the pandemic began in March 2020, the scientific community reacted with a dramatic increase in COVID-19 clinical trials, but progress in other therapeutic areas stalled due to safety concerns. The new safety and logistical challenges added by the pandemic resulted in increased costs, delays, and uncertainty in trial initiation and completion for rare disease drug manufacturers. Rare disease patients have conditions that make them more susceptible to and higher risk for COVID-19, making it especially difficult for this population to participate in clinical trials.
By October 2020, an estimated 80 percent of non-COVID trials were stopped or delayed during the pandemic. Over 700 trials experienced disruptions since the beginning of the pandemic, with over 250 suspending enrollment due to the pandemic. In May 2020, NIH Director, Dr. Francis Collins estimated that $10 billion of research outside of COVID-19 had been lost. Given the tumultuous two years that have followed, the amount of lost research has continued to multiply.
Many of the companies investing in rare disease research are small biotechnology companies that face particularly stark financial challenges, leaving them with exceedingly difficult choices on whether to resume their trials or stay in business altogether. Much of the critically necessary rare disease clinical research remains stalled, while millions of patients are waiting for breakthrough treatments and cures for the diseases that threaten their lives.
The RDCC is grateful for the leadership of Representative Josh Gottheimer (D-NJ) and Representative Don Bacon (R-Neb) to co-sponsor Leo’s Law, formerly called The Orphan Drug COVID-19 Mitigation Act, and push for its inclusion in the next available legislative vehicle. Learn more on this important issue which will help to bring hope and needed cures to the at least 95 percent of rare diseases currently lacking an FDA-approved treatment in this letter.
Executive Director Amanda Malakoff joined the Global Genes RARE Cast Podcast for a discussion with Daniel Levine. In this podcast, Amanda covers a range of topics, including why the Coalition was formed, the current policy landscape for rare disease therapies, and our policy priorities for the year ahead grounded in the continued advancement of R&D for rare disease treatments so that patients can have the best access to the treatments they need.
Listen here:
By Gabrielle Wanneh / November 22, 2022
This article was published by Inside Health Policy.
Rare disease advocacy groups hope the new Congress will prioritize investment in the development of rare disease treatments by passing bills that restore and reinforce seven-year exclusivity for drugs being developed for specific rare conditions, require regular reporting to improve the approval process of orphan drug designation and ensure patient input is counted throughout the development process.
With Republicans set to control the House and Democrats to maintain control of the Senate, and new committee leaders yet to be formally announced, it’s too early to tell how legislation aimed at accelerating the development of rare disease treatment will be handled, sources say, despite bipartisan support for some measures.
Amanda Malakoff, executive director of the Rare Disease Company Coalition, is hopeful that bills like the Better Empowerment Now to Enhance Framework and Improve Treatments (BENEFIT) Act and the Helping Experts Accelerate Rare Treatments (HEART) Act have the bipartisan support needed to be pass in one or both chambers next year.
The BENEFIT Act would make sure patient experiences, patient-focused drug development and other related data are considered as part of FDA’s risk-benefit assessment, while the HEART Act would require FDA to consult external experts and stakeholders while also providing reports on how FDA is handling applications for a drug to be designated for the treatment of a rare disease.
“There’s just a lack of expertise in general in [the area of] rare disease, so we really advocate for the FDA making sure they’re bringing people with the most timely and relevant knowledge into the process,” Malakoff told Inside Health Policy.
“Anything that can be done to streamline the process at FDA will help give that confidence back to investors on rare disease and biotech, and those two bills are just examples of steps Congress can take to help make that happen,” Malakoff added.
Another bipartisan bill, Cameron’s Law, would increase the tax credit for clinical testing expenses under the Orphan Drug Act from 25% back to the original 50%, years after the Tax Cut and Jobs Act reduced the credit in 2017. Malakoff said restoring the tax credit to its original parameters would make it more economically feasible for drug companies to develop rare disease treatments and could also send a positive message to investors.
Malakoff noted that provisions from both the BENEFIT Act and HEART Act had been included in Congress’ user fee reauthorization bill at different points in time before it passed in September. It’s unclear whether measures from either bill will make it into the upcoming year-end legislation.
Heidi Ross, vice president of Policy and Regulatory Affairs at the National Organization for Rare Disorders, said that reinforcing the seven-year exclusivity period for rare diseases and conditions under the Orphan Drug Act, and specifying that this period prohibits the approval of other drugs for the same use or indication, is another issue that may be able to garner bipartisan support. The Retaining Access and Restoring Exclusivity (RARE) Act, co-sponsored by Sens. Tammy Baldwin (D-WI) and Bill Cassidy (R-LA), would do just that.
Cassidy is expected to be the ranking Republican on the Senate health committee.
The bill would reverse the September 2021 Catalyst v. Becerra decision that upended FDA’s longstanding narrow interpretation of how and under what circumstances the exclusivity period should be granted.
“Our concern is that you will end up in a world where if you are awarding exclusivity to just cystic fibrosis, then you’re not going to have companies have the proper incentives to study those more challenging patient populations. The biggest population might be genetic mutation XYZ, but the patients that have genetic mutation ABCD also deserve to know that a therapy is safe and effective for them,” Ross said.
Similar language that codifies the FDA’s prior interpretation of how to award orphan drug exclusivity was included in the version of the Food and Drug Administration Safety and Landmark Advancements (FDASLA) Act and the Food and Drug Amendments of 2022 passed by the House passed in June. The provision ultimately did not make it into the final clean user fee package that Congress passed in September, but many of the FDA reforms that were left out of the final package could still be included in year-end legislation.
If not included in Congress’ year-end legislation, Ross said it’s also possible the RARE Act alone could garner bipartisan support next Congress should Cassidy become the ranking Republican of the Senate health committee. — Gabrielle Wanneh (gwanneh@iwpnews.com)
A recent study finds that FDA’s accelerated approval pathway can lead to faster drug development and more treatment options for patients with rare diseases that are difficult to study. Once a drug obtains FDA approval – whether through a traditional or accelerated approval pathway, patients can access the therapies through Medicaid benefits, saving lives and easing the medical burdens of many.
However, suggested proposals to limit reimbursements for accelerated approval therapies within Medicaid would limit access to—and threaten the future development of—orphan drugs that rely on this trusted pathway for viability. These proposed policy changes would cause both immediate and lasting harm to the rare disease community – and disincentivize future use of the valuable pathway for certain rare diseases.
RDCC calls on Congress to reject these unfounded proposals and instead support policies that would promote greater patient access to FDA-approved therapies.
Read more about the study’s findings and how to protect accelerated approval for rare disease treatments in this overview.
Click here to download this overview.

By: Amanda Malakoff, Executive Director, The Rare Disease Company Coalition
The state of the biotech industry is at a critical inflection point. From one perspective, people are calling this era the golden age for biotech, where cutting edge medical discoveries are showing incredible promise. For example, rare disease biotechs have recently pioneered the ability to treat patients by permanently modifying a single gene to cure disease. It’s thanks to recent medical and technological innovations like these that there has never been a more dynamic time for biotech. However, from another perspective, capital is fleeing the rare disease biotech industry, prompting concerning headlines like “Biotechs face cash crunch after stock market ‘bloodbath’.” This paradox makes it easy to see why, despite the incredible innovations being made in biotech today, it is becoming increasingly difficult to realize the full potential of promising biotech innovations.
The Rare Disease Company Coalition (RDCC) represents nearly two-dozen life sciences companies that are researching, developing, and bringing treatments to rare disease patients – often those who previously had no options available to them – thanks to breakthroughs in molecular, cell, and gene therapy. Last month, I represented the RDCC on a panel discussion on the State of the Industry at The Business of Rare Policy Summit in Washington, DC, where experts and stakeholders came together for a day of dialog.
My fellow panelists and I spoke to the challenges facing clinical stage rare disease biotechs today. Namely, without products on the market, they do not bring in revenue and are largely dependent on capital markets to fund their research. However, it’s not just clinical stage companies relying on investment for research and development (R&D). Due to the complex nature of rare diseases, a comparatively higher percentage of operating expenses is dedicated to research and development all rare disease life science companies. Commercial stage companies with treatments on the market continue to invest their profits back into R&D, spending more on research than they make in revenue. As a result, commercial-stage rare disease companies often must raise billions of dollars from investors to sustain R&D.
Unfortunately, the recent downturn in the biotech stock market is keeping promising treatments from coming to market and has left an industry once flooded with money now strapped for cash. During times of economic uncertainty, investors look for lower risk portfolios, and look to key inflection points and milestones in the pharmaceutical regulatory review process. By its nature, the rare disease drug development process incurs more risk. Treatments are manufactured for limited population sizes, but their development costs remain the same or higher than drugs for larger populations due to the lack of natural history, complex diagnosis, limited access to patients for participation in clinical trials, and the often-unprecedented regulatory pathway. Public policy has a huge impact on raising capital and getting treatments to market, yet the government’s lack of consistency in addressing these challenges is putting undue constraints on potential private investment.
Investors want certainty and often look to policies made in Washington as key indicators. A “one-size-fits-all” approach to policies like drug pricing and value assessment frameworks can be especially detrimental and destabilizing to rare drug development. Amidst the current economic headwinds, it is more important than ever for Congress to preserve and foster biotech innovation so that the gains that have been made are not lost. Supportive federal regulations and policies are sorely needed to help spawn private investment in the rare disease biotech industry.
Let’s look at hard numbers to illustrate this point. Policies like the Orphan Drug Act (ODA), which was enacted in 1983 to facilitate the development of treatments for rare and ultra-rare conditions, has had outstanding success in furthering the industry. Prior to the ODA, only 38 orphan drugs had been approved in the US, compared to over 700 today. However, in 2017, some in Congress halved the Orphan Drug Tax Credit to 25%, and significantly reduced this powerful incentive made possible under the ODA for companies to invest in cures for rare diseases. Given that less than 7% of all rare diseases have an FDA-approved treatment, we simply cannot afford to reduce incentives to find, treat and cure rare diseases.
Policies must account for the complexity of rare disease drug development, which requires substantially different – and often more costly – trial designs and business models than therapies for larger patient populations. To offset the higher risk and costs associated with rare disease research, Congress must do more to provide incentives and increased certainty for rare disease companies by strengthening policies like the Orphan Drug and R&D tax credits. Doing so would send a reassuring signal to investors and help companies secure critical funding needed to continue their important work.
Rare disease research is at a precipice where years of efforts and innovation are yielding incredible results with the potential to give patients a better chance at life. Researchers, doctors, and patients are working together to advance cutting edge medical innovation, presenting us with an extraordinary opportunity to bring hope to the more than 90% of rare disease patients that lack treatment today.
While the financial challenges facing the biotech industry are significant, the solutions are straightforward. Rare disease biotechs need investors, and investors look to decisions made in Washington to provide certainty. With so many promising therapies on the horizon, it is imperative that policymakers act now to ensure the continued success of the industry. We are on the precipice of a golden age for biotech, and to fully realize it, we must confront the reality of the market head-on and work together to create an environment that catalyzes investment for rare disease research.
See More Video Clips
Research and development (R&D) is at the heart of rare disease innovation. Due to the complex nature of rare diseases, a comparatively higher percentage of operating expenses is dedicated to R&D for the over 95% of rare diseases without an FDA-approved treatment.
In 2021, our 21 Coalition members alone invested more than $12 billion into R&D, representing on average over half of their annual budgets. In addition, many of our members with products on the market are continuing to spend more on R&D than they make in revenue, investing profits back into future treatment development. The result of these endeavors have built a remarkable hub of innovation, growth and investment across life science companies.
However, a harmful tax change that if not reversed, will hurt rare disease companies’ ability to advance lifesaving innovation while limiting additional job creation and development here in the United States. Up until January 2022, a business in the United States could deduct 100% of their R&D expenses in the year during which those expenses incurred.
Now, a change in the tax code that took effect this year requires businesses to spread those deductions over a period of years—the so-called amortization requirement—making investment in innovation more expensive to conduct.
This week, RDCC joined over 400 organizations across the greater business community to urge Congress to repeal this harmful tax change. Doing so is vital to ensure that rare disease companies can continue to innovate, advance continued and ambitious research and development programs, and bring treatments to patients. On the cusp of significant breakthroughs in molecular, cell and gene therapy advancements, we must continue to support medical innovation taking place at rare disease companies to support delivery of life-altering treatments to rare disease patients.
Read the full letter to Congressional leadership here.

The Rare Disease Company Coalition (RDCC) submitted comments on S. 4348, the Food and Drug Administration Safety and Landmark Advancements (FDASLA) Act of 2022. In a follow-up letter to the House Energy & Commerce (E&C) and Senate Health, Education, Labor, and Pensions (HELP) Committees, the RDCC writes to commend the work done to date while strongly encouraging the timely reauthorization of the user fees to ensure continuity of critical funding for the Food and Drug Administration. Timely action will avert a delay in the rare disease drug review process.
Please see here for the RDCC’s letter.

Our Coalition joined with more than 70 organizations, led by the March of Dimes, to call on the U.S. Senate to advance the “Newborn Screening Saves Lives Reauthorization Act of 2021” (S. 350). The House version of the bill (H.R. 482) passed with overwhelming bipartisan support last summer. Given the very limited availability on the Senate calendar, now is the time for the Senate to act on this bill and advance it to the floor.
Each year thousands of babies are born with rare diseases that may not be apparent at birth. Fifty years ago, these infants’ disorders would have gone undetected until symptoms appeared, leading to possible death or lifelong disability. Today, a simple set of newborn screening tests performed at birth can detect these life-threatening illnesses before any symptoms begin, allowing crucial time for early diagnosis and treatment.
Click here to read the full letter submitted to Congressional leadership.

Newborn screening is a vital and proven public health program that screens approximately four million U.S. newborns each year. In the first 24 to 48 hours of a baby’s life, a small blood sample is taken to detect serious, often fatal, genetic conditions that can be treated if diagnosed early. We believe equitable access to newborn screening can ensure that all babies have the best chance for a healthy life. Join us in working towards a modern newborn screening system that provides equal access to a timely diagnosis for all babies.
Learn more in this educational resource.

RDCC submitted comments to Board Members of the Colorado Prescription Drug Affordability Review Board (PDAP) concerning the impact a proposed rule could have on advancing research and development for new and innovative rare disease therapies. The Coalition asserted that the draft proposed rule as written could “in the long run harm more patients than it helps.”
In this letter, the RDCC strongly urges the PDAB to consider the unique circumstances of rare disease patients and therapies as it crafts its policy proposals, and cautions against punitive measures that would have an outsized impact on rare diseases. The impact of misguided policy proposals could stall future investment and development of rare disease treatments that would further disadvantage rare disease patients.

| A “one-size-fits-all” approach to drug pricing and value assessment frameworks undermines development of therapies for people – very often children – with rare diseases. As rare disease companies, we champion policies that help people with high unmet needs by developing innovative cutting-edge medicines and working with federal and state policymakers to address access and affordability. Learn more about the importance of restoring equity and patient focus when valuing rare disease treatments in this overview. |

Washington, DC – June 6, 2022 – The Rare Disease Company Coalition (RDCC), a unified voice of life science companies committed to discovering, developing and delivering rare disease treatments, recently welcomed Amanda (Schechter) Malakoff as its next executive director. Malakoff started in her new role May 31, 2022, and will be a key spokesperson for the RDCC and an advocate for companies dedicated to rare disease treatment development.
“Amanda brings a terrific blend of experience in association management and working on member companies’ legislative and regulatory issues,” said John Jackimiec, chair of the RDCC. “She will play a critical role in extending the reach of our coalition and advancing the policy priorities of the RDCC through our education and advocacy work. We are all looking forward to her leadership and contributions.”
Prior to joining the RDCC, Malakoff was senior director of member relations for the National Association of Manufacturers (NAM), where she was responsible for representing the policy interests of mid-size manufacturing companies and advising industry executives on government relations strategies. Malakoff previously held client relations roles at Bloomberg Government and the Advisory Board Company, working with pharmaceutical and healthcare leaders on legislative and regulatory monitoring as well as operational best practices. She also worked on Capitol Hill in the office of Congressman John Carney, Jr. Malakoff will graduate in 2023 with a master’s in public administration from the George Washington University’s Trachtenberg School of Public Policy and Public Administration with a focus in regulatory policy.
With a diverse and growing membership, the RDCC informs key policy stakeholders and members of Congress on the unique considerations of life science companies when developing and manufacturing rare disease therapies as well as the importance of improving patient access following U.S. Food & Drug Administration (FDA) treatment approval. The Coalition believes that constructive dialogue with well-informed policymakers will lead to policies and regulations that enable continued innovation and the timely development and commercialization of treatments for the one in 10 Americans living with rare diseases, half of whom are children.
“I am thrilled to join the RDCC during this pivotal time of growth and impact, and honored to step into the position of executive director,” said Amanda Malakoff, executive director of the RDCC. “With more than 90% of rare diseases without a viable treatment, there is an urgent opportunity to advocate for the needs of life science companies working to deliver hope to patients.”
Over the past year, the RDCC has experienced growth in both size and scope. Collectively, RDCC represents 21 company members, totaling $12.4 billion in research and development investments for rare disease treatments. The companies are united around a shared mission to improve the lives of people living with rare diseases.
About the Rare Disease Company Coalition (RDCC)
Founded in May 2021, the Rare Disease Company Coalition represents life science companies committed to discovering, developing and delivering rare disease treatments for the patients we serve. As an education and advocacy-focused coalition of companies, our goal is to inform policymakers of the unique challenges and promises of rare disease drug discovery, development and manufacturing for small population sizes so that critical innovation can continue and positive changes can be enacted for the rare disease community. To achieve this goal, we will use our unified voice to advocate for long-term, consistent, equitable and sustainable government policies that enable life science companies to continue to bring hope and provide access to approved treatments to people living with rare diseases. For more information, please visit rarecoalition.com
Media Contact:
Anna Stallmann
media@rarecoalition.com
708-476-1258
By: Dr Neil Thompson, Chief Scientific Officer at Healx, a company member of the Rare Disease Company Coalition
Innovation in the biopharma industry isn’t always about the development of a new therapy for a condition – sometimes it means pioneering an entirely new approach. Right now, we’re witnessing exactly that, as artificial intelligence (AI) promises to re-engineer the entire drug discovery and development process.
Indeed, AI and other new approaches are allowing novel treatments to be designed, developed and delivered more quickly and on a scale never before seen in the industry. This is offering hope to millions of patients in need – particularly those living with a rare condition (95% of which still lack an approved treatment today).
But what are the implications of this technology for policy, patients and treatment pipelines?
How did we get here?
The historical phenotypic-based and target-based approaches of drug discovery have delivered many of the life-saving and life-changing treatments that health systems now have available to them. Yet, despite the successes, the philosophy of ‘one disease, one target, one drug’ has its limitations.
Traditionally, drugs have been developed to target a single biological entity, and it was viewed as undesirable for a drug to interact with multiple targets due to possible adverse side effects. But this approach often fails complex medical conditions, especially many rare diseases which exhibit a broad range of symptoms and causes.
Increasingly, there is evidence that a molecule hitting more than one target, or multiple molecules targeting a single disease, can create a more efficacious profile compared to single-targeted molecules. However, whilst we are seeing a slight increase in the number of multi-target therapies being approved by the FDA (21% of all FDA-approved agents between 2015-2017 were multi-target drugs), the majority of drugs being studied and in line for approval are still addressing a single target.
This poses a problem for rare diseases not only for the biological reasons mentioned above, but also because policy focused on single target therapies continues to steer therapy development towards common diseases – where there are larger patient populations from which to recoup R&D costs – meaning rare conditions remain overlooked.
Policy for progress
Advances in AI in the last decade have helped improve core processes within the drug discovery and development pipeline – from predicting protein structures and discovering new compounds, to monitoring patients during clinical trials and shaping go-to-market strategies.
AI is particularly adept at tackling the conventional challenges of rare disease treatment development. For example, Natural Language Processing (NLP) models can overcome the lack of consolidated knowledge about a rare condition by automatically scanning literature to fill in the gaps with up-to-date data. Machine Learning (ML) algorithms can speed up the drug discovery and development timeline by looking for connections between diseases and the pharmacology of compounds that are already approved for use in another condition, thereby providing valuable insight into targetable mechanisms for diseases that are still poorly understood biologically. And AI can provide the automation and scale needed to provide cost-effective treatments, thereby reducing the cost of R&D for smaller patient populations.
But therapies for rare diseases can only be developed more efficiently and cost-effectively when policy and regulation keeps up with technology. Promisingly, progress is being made in this space.
The Orphan Drug Act of 1983 ushered in a new energy to focus on rare diseases, granting financial and regulatory incentives for the biotech and pharmaceutical industry to develop treatments for them. The 21st Century Cures Act also provided additional incentives and regulatory changes, and policies like the Orphan Drug Tax Credit and the Accelerated Approval Pathway help improve the likelihood of investment in the rare disease space in order to catalyse the therapy development process and increase the likelihood of therapies reaching patients.
Then there are groups like the Rare Disease Company Coalition, who are championing further changes in policy and regulation to support technological advances in rare therapy development.
Just the beginning
What makes AI and other technological advances so fascinating is that as computing power and improvements to data quality are made, they can be rapidly scaled up. The solutions will come faster and faster as biotechs become able to automate more of the process and run more of it in parallel, with expert humans placed along the chain, at decision points where they can provide maximum impact.
It’s not hard to imagine a future where we’re able to discover treatments for whole groups of conditions at once, guided by both technological and human insight. Suddenly, no disease, however rare, need be overlooked, no question about biology left unanswered. But policies and regulations need to be in place to support these advances, so that tech-derived therapies can reach patients more quickly, especially in the rare disease sector.
Pharmacology isn’t just another discipline for AI to be applied to. It could be the most disruptive transformation in the 21st century. It is essential that government policies continue to keep pace with innovation so that novel uses of technology, including AI, can be readily deployed and utilized across treatment discovery and development to better support people with rare diseases.
We are on the cusp of great progress, we can’t afford to be slowed down now.
Bringing over 30 years experience in drug discovery and development, Neil leads Healx’s preclinical work and is deeply passionate about the application of AI to improve rare disease patient access to treatments.
| The Orphan Drug Tax Credit is a vital lifeline for rare disease patients and should be protected to preserve hope for the rare disease community. Given that approximately 95% of rare diseases of rare diseases lack an FDA-approved drug, any proposal to reduce the ODTC would have a devastating impact on orphan drug development in the U.S. and on millions of Americans living with a rare disease. We must preserve innovation and not lose the gains that have been made since the passage of the ODA. Learn more about the Orphan Drug Tax Credit and its role in rare disease treatment development in this overview. |
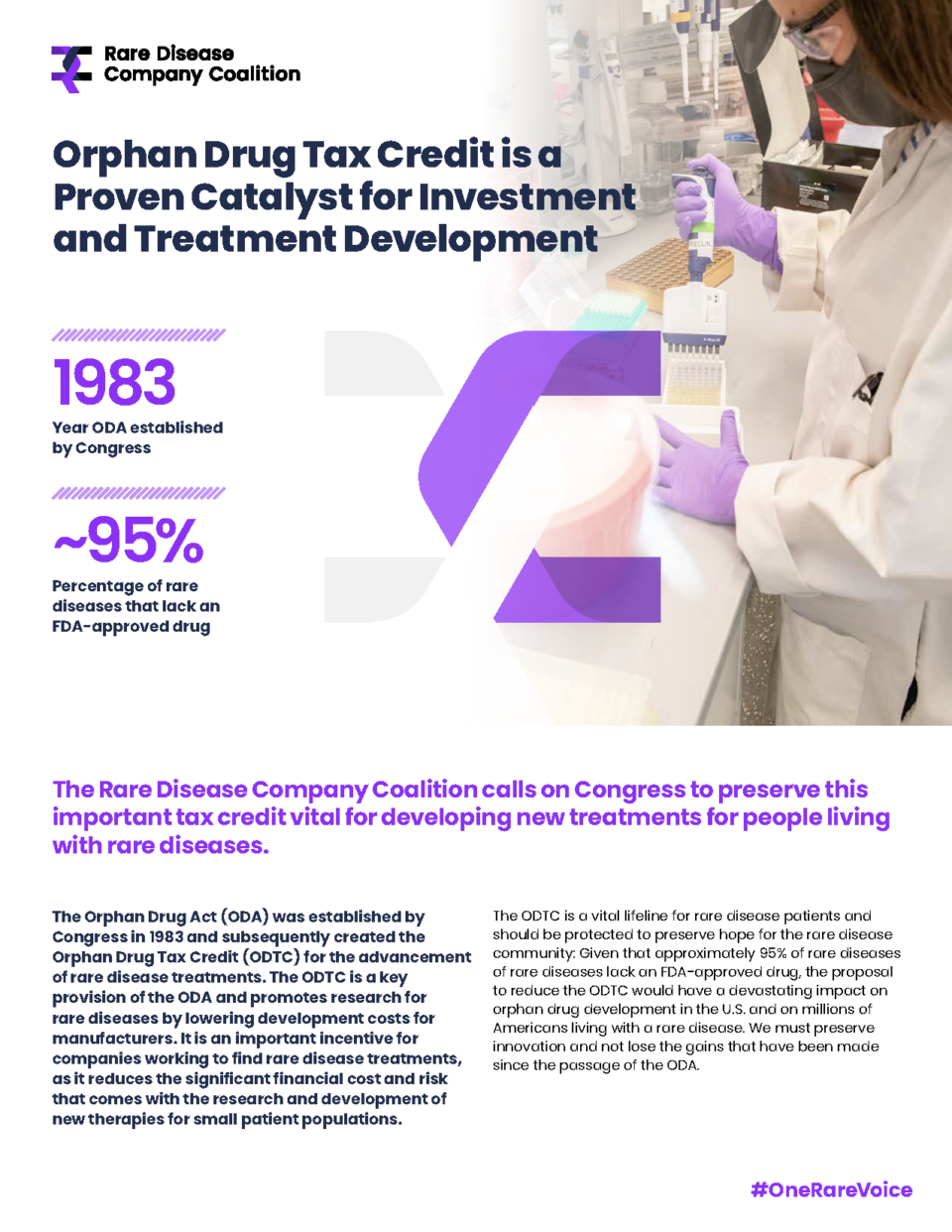
| FDA’s accelerated approval pathway recognizes that a “one-size-fits-all” traditional model cannot always work – especially for serious and unique rare diseases with very small population sizes that progress slowly and variably from patient to patient. Therapies approved through the accelerated approval pathway are subject to the same stringent, evidence-based clinical review and approval standards as the traditional FDA approval process, as well as to post-approval confirmatory trials to verify clinical benefit. While less than 10% of all accelerated approvals in its 30-year history have been used for nononcology rare diseases, today’s increased understanding of rare disease and advances in targeted drug development are making it possible to realize the promise of accelerated approval for rare diseases where current treatments don’t yet exist. As policymakers consider policy proposals that impact the trajectory of rare disease treatment development, we advocate for an approach that considers the unique circumstances of rare diseases and caution against punitive measures that could chill future investment and development of rare disease treatments. |
Click here to download this overview on the benefits of the accelerated approval pathway for rare diseases.

Building on our letter submitted to the Oregon Health Authority (OHA) on the proposed renewal and amendment of its Section 1115 waiver, RDCC submitted comments to the Department of Health and Human Services Secretary Becerra and Centers for Medicare and Medicaid Services (CMS) Administrator Brooks-LaSure on the OHA Section 1115 Demonstration Waiver. In this letter, the RDCC expresses concern with the potential impact that waiving certain provisions of Section 1927 of the Social Security Act would have on the discovery and development of new medicines, particularly on treatments and cures for patients with rare diseases who currently have limited or no treatment options.
Limiting Medicaid coverage for viable treatments would have a devastating impact on patients benefiting from this innovation and create a greater healthy equity divide, all while resulting in limited budget savings. This proposal is the wrong solution for patients.

The Rare Disease Company Coalition (RDCC) is proud to support the bipartisan Better Empowerment Now To Enhance Framework And Improve Treatments (Benefit) Act (H.R. 4472 / S. 373). We applaud the work Congress has done to date to advance patient engagement in drug development and this legislation introduced by Representatives Doris Matsui (CA-6) and Brad Wenstrup (OH-2) and Senators Roger Wicker (R-MS) and Amy Klobuchar (D-MN) demonstrates a continued interest in doing so.
The BENEFIT Act addresses the need to reflect the patient perspective and experience in the FDA’s risk-assessment tool. This policy would play a critical role in the FDA evaluation and approval decision regarding drugs and other medical products and will allow the FDA to capture meaningful patient experience, PFDD, and related data as part of the benefit-risk assessment.
The RDCC believes this legislation will advance patient engagement, which ultimately can help advance the development and delivery of
treatments to those living with rare diseases. We applaud Rep. Matsui, Rep. Wenstrup, Sen. Wicker and Sen. Klobuchar for championing policies to improve the regulatory process and supporting the voice of the rare disease patients we serve.
Read our full endorsement letter here.

Why a whole-of-society commitment makes economic sense
By: Gina Cioffi, Senior Manager of Public Affairs at Chiesi Global Rare Diseases, a company member of the Rare Disease Company Coalition. This article was written in response to a new report – “The Burden of Rare Diseases: An Economic Evaluation” – authored by experts at IQVIA and Chiesi Global Rare Diseases.
Rare diseases represent a major societal issue with enormous costs and without a clear, concentrated, whole-of-society effort to address the burden on patients, families and caregivers.
At Chiesi Global Rare Diseases, we set out to studycost drivers contributing to the burden of rare diseases to determine the societal benefit of early diagnosis and availability of pharmaceutical treatments. Along with a number of recent publications on the societal and economic burden of rare diseases, our results concur that the magnitude of the cost burden is staggering. The full report – “The Burden of Rare Diseases: An Economic Evaluation” – is available for download.
We know that lack of rare disease data compromises care and adds complexity to the innovation and drug approval process. And, the full extent of the patient, family, and societal burden will remain mostly undocumented. Our study was done through systematic review of the literature as well as expert consultation. We sought to identify new ways to better understand the cost drivers and economic impact that a lack of available treatments poses. This endeavor is critical so that policymakers can understand the benefit of investing in pharmacologic innovation, and it will encourage the development of policies to accelerate the availability of, and access to, rare disease treatments.
Discussions with patient advocacy organizations and physicians led to the selection of 24 rare diseases, each with high unmet needs, across five therapeutic areas including metabolic, neurological, congenital, hematological, and immunological. The selection criteria included the degree of unmet need, relative importance to patient advocacy organizations, interest in the scientific community, prevalence, and apparent burden of disease.
Our assessment consideredthe direct, indirect, and mortality-related costs of the selected 24 rare diseases. Direct costs include treatment, medical procedures, hospitalizations, physician visits, home healthcare, and other medical costs. Indirect costs include patient and caregiver productivity loss, work loss, home changes, and traveling and accommodation for medical visits. Mortality costs are based on value of statistical life (VSL) and the difference between average life expectancy and that for people with a rare disease.
Among our findings:
- The average overall economic burden per patient per year (PPPY) is $266,000, which is approximately 10x the cost associated with mass market diseases.
- In the detailed analysis of the selected 24 rare diseases impacting 584,000 people in the US, the total cost to society is approximately $125 billion.
- The cost burden for 8.4 million patients in the US impacted by 373 rare diseases considered in our broader analysis is estimated to be $2.2 trillion per year if treatments were available and $3.9 trillion per year if no treatments were available.
- The value returned to society if treatment were available for these 373 rare diseases is $1.7 trillion per year.
- Considering National Institutes of Health (NIH) estimates of 25–30 million Americans with a rare disease, the burden range is estimated to be $7.2 trillion to $8.6 trillion per year.
Although most direct costs are covered by government and commercial insurers, substantial indirect and out of pocket expenses impact patients and their families. We found that cost of rare diseases would be, on average, 21% more expensive PPPY if there was no access to treatment, demonstrating further that therapy access can alleviate government and family burden.
Keep in mind that these overall findings may represent an underestimation. Social costs, including the impact on health-related quality of life, were not part of this analysis.
A major strength of this study is that it presents an economic tool for analysis of the positive impact of rare disease treatments. This data can help justify increased governmental investment to ensure wider patient access to therapies and policy proposals that are reflective of the unique challenges rare disease patients and companies face.
Immediate actions that can accelerate access to treatment include passage of the Newborn Screening Saves Lives Act, providing the Centers for Disease Control and Prevention (CDC) funding to increase its support for State Newborn Screening programs, and full funding of the Food and Drug Administration (FDA) Orphan Products Grant Program. Further focus to ensure legislative efforts prioritize and reflect the unique circumstances and needs of rare disease patients is another imperative.
Incentives for drug development, particularly the Orphan Drug Tax Credit (ODTC), have changed the trajectory for many rare disease patients and likely achieved significant reduction in federal spending and overall burden. Prior to the ODTC, the number of annual deaths from rare diseases was growing at a slightly higher rate than that from other diseases (2.0 percent versus 1.3 percent, respectively). In the 10 years following, the number of annual deaths from rare diseases declined at a rate of 3.1 percent, while the annual number of deaths from other diseases continued to grow at a rate of 1.2 percent. And, prior to the ODTC, only 38 drugs were FDA approved for rare diseases. Today more than 650 rare disease drugs have been approved by the agency.
Despite this success, the ODTC was targeted in the 2017 Tax Cut and Jobs Act when Congress reduced the total amount of the tax credit for qualifying clinical testing expenses from 50 percent to 25 percent. Our industry has yet to see the full impact of that reduction, and yet in the Build Back Better Act we faced another devastating reduction to the tax credit.
Our hope is that policymakers will continue to nurture and sustain innovation based on the positive economic return from rare disease therapies.
The bottom line is that access to therapies for people living with rare diseases generates significant value for society. Now is the time for the Administration and Congress to come together with rare disease stakeholders and implement actions prioritizing the unmet needs of 30 million Americans impacted by rare diseases.
Click here to download an overview of our Policy Focus Areas.

By: Dr. Jason Shafrin of FTI Consulting. This op-ed was written in response to a new white paper published by Alexion, AstraZeneca Rare Disease, a company member of the Rare Disease Company Coalition.
Although individual rare diseases have a low prevalence, one in 10 Americans is affected by one of 7,000 known rare conditions. Approximately 37 percent of rare disease patients have reduced life expectancies due to a lack of treatment options for conditions, and currently, 95 percent of rare diseases have no treatment option.[i]
Legislation, such as the Orphan Drug Act, helped increase the number of rare disease treatment options available; however, the steadily rising costs for these treatments are a cause of concern for lawmakers. As a result, policy experts are calling for a health technology assessment (HTA) framework that would determine whether the value of a pharmaceutical warrants its price.[ii]
Despite intentions to curb rising health care costs, the implementation of an HTA framework could stifle the balance of innovation in and access to orphan drugs. Research from FTI Consulting’s Center for Healthcare Economics and Policy on how orphan drugs would be affected under an HTA framework finds that the HTA framework could negatively impact innovation, development and access to orphan drugs. This is due to a tendency for many HTAs to apply a one-size-fits-all framework to orphan drugs for rare diseases, which inherently have unique needs and are high-risk endeavors.
The research finds that adopting a national HTA body based on the practices of the Institute for Clinical and Economic Review (ICER) could severely curtail access to orphan drugs for rare diseases.[iii] The millions of patients in the United States who already struggle to gain access to treatment for rare conditions cannot afford to lose even more access to treatment. For example, a recently approved therapy for spinal muscular atrophy underwent significant approval delays in the United Kingdom (among other countries) because the UK’s HTA body—known as NICE—determined that the price was too high to justify the treatment gains, despite robust clinical evidence that the drug showing a “substantial benefit.”[iv] Even for those on treatment, rare disease patients can benefit from improved innovations that help enable them to live longer, fulfilling lives.
Many of the value assessments conducted by current HTA bodies fail to account for broader societal benefits orphan drugs provide nor do they take into account how orphan drugs could reduce health disparities. Few HTA bodies value assessments framework incorporate value from allowing patients to return to work or school, or whether new treatment reduce the burden on parents caring for children with rare diseases. Impacts on health disparities are also largely ignored within traditional value assessment and patient advocates are concerned that negative HTA assessments will impact access. Regarding ICER’s negative assessment of new myasthenia gravis treatments, one patient advocate group stated that any barriers to care, such as ICER’s negative evaluation, “… will likely further disproportionately impact Black patients and exacerbate health inequities”.[v]
Looking ahead, applying an HTA framework to orphan drugs for rare diseases could not only hamper access to existing rare disease treatments, but also it could also decrease the likelihood that new orphan drugs will be introduced to the market. Such as the Congressional Budget Office have found that government price controls—whether due to HTA value assessment or other sources—would significantly reduce the number of new treatments that are brought to market.[vi]
As Congress is assessing current health care initiatives and programs for the future, now is an important opportunity to examine how access to rare orphan drugs are impacted through assessment under the national health technology assessment framework.
As the research points out, a one-size-fits-all model has potential negative consequences for access to and development of orphan drugs. Industry leaders, policymakers, and patient advocates can use this research as a starting point for a timely discussion on how to preserve access and innovation for orphan drugs. In order to properly address potential negative outcomes of all frameworks, a discussion is the first step. Now more than ever, it is imperative that we convene all stakeholders to build on this research. Millions of Americans living with rare diseases are depending on it.
The views expressed herein are those of the author(s) and not necessarily the views of FTI Consulting, Inc., its management, its subsidiaries, its affiliates, or its other professionals.
FTI Consulting, Inc., including its subsidiaries and affiliates, is a consulting firm and is not a certified public accounting firm or a law firm.
_______________
[i] Stoller, James K. “The Challenge of Rare Diseases.” ScienceDirect, June 2018.
[ii] Lakdawalla, Darius et al., “Health Technology Assessment in the U.S.-A Vision for the Future.” USC Schaeffer. February 9, 2021. https://healthpolicy.usc.edu/research/health-technology-assessment-in-the-u-s-a-vision-for-the-future/
[iii] FTI Consulting, “Challenges in Preserving Access to Orphan Drugs Under an HTA Framework.” December 2, 2021.
[iv] Taylor, Phil. “Shock as NICE Turns Down Biogen’s SMA Therapy.” PMLive, August 14, 2018. http://www.pmlive.com/pharma_news/shock_as_nice_turns_down_biogens_sma_therapy_1248819.
[v] “ICER Myasthenia Gravis Public Comment.” ICER. ICER, April 2, 2021. https://icer.org/wp-content/uploads/2021/03/ICER_Myasthenia-Gravis_Public-Comment-Folio_091021.pdf.
[vi] Congressional Budget Office. CBO’s Simulation Model of New Drug Development. August 2021. https://www.cbo.gov/system/files/2021-08/57010-New-Drug-Development.pdf
Our Coalition joined with more than 80 organizations, led by the March of Dimes, to call on policymakers to include the “Newborn Screening Saves Lives Reauthorization Act of 2021” (H.R. 482) as part of the FY22 omnibus spending bill in support of improving the health of babies born with rare diseases.
Each year thousands of babies are born with rare diseases that may not be apparent at birth. Fifty years ago, these infants’ disorders would have gone undetected until symptoms appeared, leading to possible death or lifelong disability. Today, a simple set of newborn screening tests performed at birth can detect these life-threatening illnesses before any symptoms begin, allowing crucial time for early diagnosis and treatment.
Click here to read the full letter submitted to Congressional leadership.
Contact your member of Congress by clicking here.

WASHINGTON – February 3, 2022 – The Rare Disease Company Coalition, a unified voice of life science companies committed to discovering, developing and delivering rare disease treatments, today announced appointments to its Executive Committee for 2022, serving a one-year term.
With a diverse and rapidly growing membership, the Coalition informs key policy stakeholders and members of Congress on the unique considerations of life science companies when developing and manufacturing rare disease therapies as well as the opportunities for improving patient access following FDA treatment approval. The Coalition believes that constructive dialogue with well-informed policymakers will lead to policies and regulations that enable continued innovation and the cost-effective and timely development and commercialization of treatments for the one in 10 Americans living with rare diseases, half of whom are children.
The Coalition is governed by a Board of Directors and supported by an Executive Committee that provides oversight of the day-to-day operations of the Coalition. In addition to named elected positions, the Executive Committee has representation from both commercial and pre-commercial Coalition members.
The following company representatives were elected by the Board of Directors to serve as Executive Committee members for the 2022 calendar year of the Coalition:
- Chair: John Jackimiec, Aeglea BioTherapeutics
- Vice Chair: Deirdre Parsons, Alnylam Pharmaceuticals
- Secretary: Betsy Ricketts, Ultragenyx
- Member-at-Large: Diane Berry, Sarepta Therapeutics
- Member-at-Large: Christine Harrison, Orchard Therapeutics
“I am honored to represent the Rare Disease Company Coalition as we embark on our second year and build on our successes from 2021,” said John Jackimiec, newly appointed Chair of the Rare Disease Company Coalition. “In 2022, the Coalition can maximize our impact through an increase in membership, improving our reach at both the national and state level to ensure that national- and state-level policies support continued innovation for the millions of patients and families affected by rare diseases.”
In addition, the following individuals have been appointed Committee Chairs for 2022:
- Federal Policy Committee Co-Chairs: Katie Jones, Alexion Pharmaceuticals; Geoff Werth, Harmony Biosciences
- State Policy Committee Chair: Kate Segal, Sarepta Therapeutics
- Communications Committee Chair: Jessi Rennekamp, Agios Pharmaceuticals
- Operations Committee Chair: Bruce Bloom, Healx
To learn more about the Coalition and the need for informed policy and legislative discourse and actions related to rare disease treatment development and commercialization, please visit rarecoalition.com.
About
The Rare Disease Company Coalition represents life science companies committed to discovering, developing and delivering rare disease treatments for the patients we serve. As an education and advocacy-focused coalition of companies, our goal is to inform policymakers of the unique challenges and promises of rare disease drug discovery, development and manufacturing for small population sizes in order for critical innovation to continue. To achieve this goal, we will use our unified voice to advocate for long-term, consistent, equitable and sustainable government policies that enable life science companies to continue to bring hope and provide access to approved treatments to people living with rare diseases. For more information, please visit rarecoalition.com
Earlier this month, our Coalition expressed concern to the Oregon Health Authority (OHA) on the proposed renewal and amendment of its Section 1115 waiver. We are a Coalition of biotech companies dedicated to the research, development and delivery of innovative treatments for rare diseases with over 225 rare disease development programs in process, we are deeply concerned about the impact of the proposed closed formulary provisions on the rare disease community. The proposal would limit coverage to one drug per therapeutic class and exclude drugs approved via the accelerated approval pathway. 93% of rare diseases do not have a single FDA approved treatment and limiting coverage for Medicaid beneficiaries that live with a rare disease jeopardizes patient health while threatening to curtail innovation.
We urge our Oregon-based partners, advocates, individuals living with rare diseases, and their caregivers to contact the Governor and their state legislators to voice their concern with this proposed amendment. Limiting Medicaid coverage for viable treatments would have a devastating impact on patients benefiting from this innovation and create a greater healthy equity divide, all while resulting in limited budget savings. This proposal is the wrong solution for patients.
Contact Information:
Governor Kate Brown
Submit comments online at: https://www.oregon.gov/gov/Pages/share-your-opinion.aspx
Call directly at (503) 378-4582
To find your state legislator, please visit https://www.oregonlegislature.gov/findyourlegislator/leg-districts.html
Click here to download the letter submitted from our Coalition to OHA on January 7, 2021.

By: Aseel Bin Sawad, Pharm D, MSc, MCR, MS, PhD, DBA, Global Reimbursement and Health Economics Lead at Aeglea Biotherapeutics, a company member of the Rare Disease Company Coalition
There are 7,000 to 10,000 rare diseases when looking at all rare diseases in a collective manner.1 Around 25 to 30 million Americans suffer from rare diseases which equates to 1 in every 10 individuals and 50% are children.2-4
Many rare diseases are life-threatening and progressive with substantial morbidity and early mortality.5,6 Unfortunately, only 5 to 7% of rare diseases have FDA-approved treatments, and the majority of these treatments (75%) treat only one rare disease.7
It is imperative to understand the unique challenges of the entire rare disease ecosystem to provide cost-effective and timely access to treatments for people living with rare diseases.
The Challenges
Challenges associated with rare diseases include, but are not limited to, the ability to make an accurate and timely diagnosis; overwhelming clinical burden; lack of adequate real-world data; ability to quantify the economic burden; and pricing and access.
Diagnoses that are Delayed, Undiagnosed and/or Misdiagnosed
It is common for rare diseases to go undiagnosed or misdiagnosed for years, leading to serious complications such as brain damage, intellectual disability, physical disability, among others. Many rare diseases are associated with a poor quality of life due to their progressive nature, extensive utilization of healthcare resources to manage symptoms, and a substantial economic burden on the patients, caregivers and healthcare system.5,6 A recent study shows that diagnostic journeys of patients with rare diseases are lengthy and delay in diagnosis results in advanced, irreversible, and costly complications.8 Despite being born with the condition (i.e., genetic rare disease), one study reports that patients were not accurately diagnosed until an average age of 6.4 years.9
Inherited metabolic disorders are good examples of the unique challenges posed by rare diseases. While affected infants can be diagnosed through newborn screening (NBS), there are limitations. NBS is not available for most rare diseases, and for those that are included in NBS, the availability and reliability may vary from state to state. In some cases, there is lack of consistency in using appropriate analytical cut-off values for disease indicators.10,11 In addition, false negative results can occur because some plasma enzymes may take time to accumulate in newborns.12
Another challenge during the diagnosis of rare diseases is the heterogeneity of the symptoms. You can find two siblings having the same rare disease, but they have different symptoms. Similarly, many rare diseases often share similar symptomatology, leading to a misdiagnosis. Due to the extremely low prevalence of certain rare diseases, even the ‘disease experts’ may only see a handful of cases in their entire career.
Misdiagnosis can also result in a delayed diagnosis, but with extra clinical and economic burden. Patients may experience serious clinical adverse events due to medications prescribed for a disease that they do not have. In addition, further utilization of healthcare resources such as physician visits, emergency room visits, and hospitalizations, leads to extra economic burden (on the patients, caregivers and healthcare system) while patients continue the progression of their underlying, actual disease.
These examples visualize the differences between a patient who was diagnosed with a rare disease at the right time (Case 1), a patient who was not diagnosed (Case 2), a patient whose diagnosis was delayed (Case 3), and a patient who was misdiagnosed (Case 4).
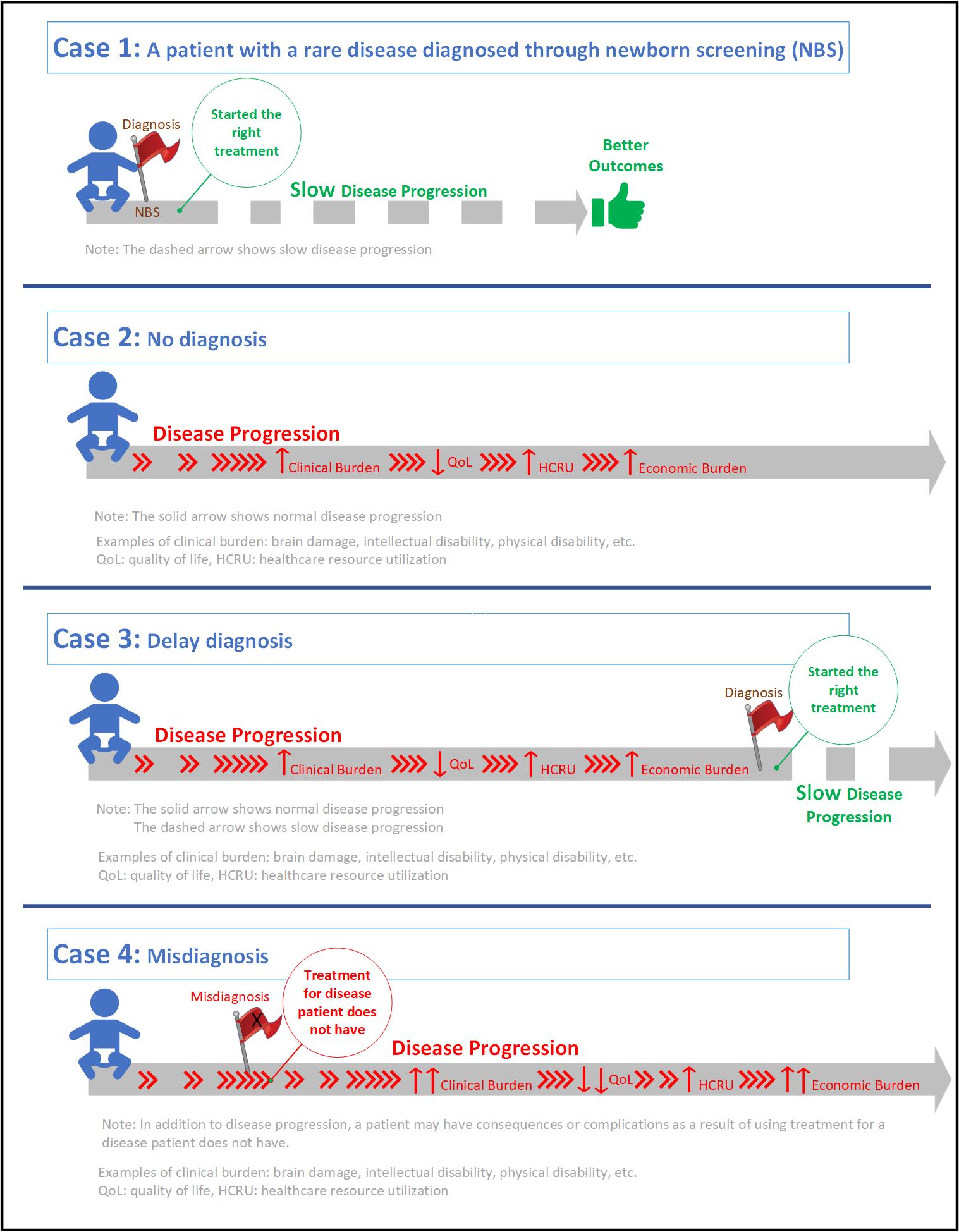
We call on policymakers to make informed decisions when it comes to rare diseases, to support and expand early diagnosis programs like NBS and timely patient access to orphan drugs to enable better health outcomes.
Congress must also preserve and increase incentives to continue attracting, encouraging, and enhancing long-term investments and innovations of rare disease diagnostics and treatments. It is less expensive for the healthcare system to diagnose a patient on time versus risking complications or a massive event where a child, or others, ends up needing long-term care through state aid.
As we move into 2022, we must look at the big picture to ensure that rare disease patients live the quality of life they deserve, and receive the right diagnosis, at the right time, with the right support.
References
- Haendel M, Vasilevsky N, Unni D, Bologa C, Harris N, Rehm H, Hamosh A, Baynam G, Groza T, McMurry J, Dawkins J, Rath A, Thaxon C, Bocci G, Joachimiak MP, Kohler S, Robinson PN, Mungall C, Oprea RI. How many rare diseases are there? Nat Rev Drug Discov. 2020;19(2):77–8. https://doi. org/10.1038/d41573-019-00180-y.
- Institute of Medicine (IOM). Chapter 2. Profle of rare diseases. IOM (US) committee on accelerating rare diseases research and orphan product development. In: Field MJ, Boat TF (eds). National Academies Press (US), Washington, DC; 2010. https://doi.org/10.17226/12953.
- NCOD (National Commission on Orphan Diseases). Report of the national commission on orphan diseases. Public Health Service, U.S. Department of Health and Human Services, Rockville, MD; 1989.
- NIH NCATS. Genetics and rare diseases information center. FAQs about rare diseases. https://rarediseases.info.nih.gov/diseases/pages/31/faqs- about-rare-diseases. Accessed 06 November 2021.
- Klimova B, Storek M, Valis M, Kuca K. Global view on rare diseases: a mini review. Curr Med Chem. 2017;24:3153–8. https://doi.org/10.2174/09298 67324666170511111803.
- Ryder S, Leadley RM, Armstrong N, Westwood M, de Kock S, Butt T, Jain M, Kleijnen J. The burden, epidemiology, costs and treatment for Duchenne muscular dystrophy: an evidence review. Orphanet J Rare Dis. 2017;12:79. https://doi.org/10.1186/s13023-017-0631-3.
- NORD (National Organization for Rare Disorders). A NORD® Commissioned Report with Avalere®. Orphan drugs in the United States: An examination of patents and orphan drug exclusivity. 2021. https://rarediseases.org/wp-content/uploads/2021/03/NORD-Avalere-Report-2021_FNL-1.pdf. Accessed 07 November 2021.
- Tisdale A, Cutillo CM, Nathan R, Russo P, Laraway B, Haendel M, Nowak D, Hasche C, Chan CH, Griese E, Dawkins H. The IDeaS initiative: Pilot study to assess the impact of rare diseases on patients and healthcare systems. Orphanet J Rare Dis. 2021;16(1):1–8. https://ojrd.biomedcentral.com/track/pdf/10.1186/s13023-021-02061-3.pdf
- Bin Sawad A, Pothukuchy A, Badeaux M, Hodson V, Bubb G, Lindsley K, Uyei J, Diaz GA. The natural history, clinical outcomes and unmet needs of patients with Arginase 1 Deficiency (ARG1-D): A systematic review of case reports. Value in Health. 2021 Jun 1;24:S4.
- Cederbaum S, Therrell B, Currier R, Lapidus D, Grimm M. Newborn screening for arginase deficiency in the U.S. – Where do we need to go? Paper presented at: ACMG Annual Clinical Genetics Meeting2017.
- Therrell BL, Currier R, Lapidus D, Grimm M, Cederbaum SD. Newborn screening for hyperargininemia due to arginase 1 deficiency. Mol Genet Metab. 2017;121(4):308-313.
- Jay A, Seeterlin M, Stanley E, Grier R. Case Report of Argininemia: The Utility of the Arginine/Ornithine Ratio for Newborn Screening (NBS). JIMD Rep. 2013;9:121-124.
By: Betsy Ricketts, Chair of the Rare Disease Company Coalition, and Vice President of Policy, Government and Public Affairs at Ultragenyx, a company member of the Rare Disease Company Coalition
Originally Published at TheHill.com (December 29, 2021)
Congress’s proposed limits to the Orphan Drug Tax Credit (ODTC) in the Build Back Better Act (H.R. 5376) could severely hurt development of potential treatments for rare diseases that impact 30 million people in the United States, half of whom are children. It is difficult to understand how Congress could agree to the drastic reduction of this proven resource that supports the development of new treatments for 90 percent of the approximately 7,000 known rare diseases that currently do not have an FDA-approved treatment, many of which are life-limiting or fatal.
To understand the significance of the ODTC, it is important to understand the unique challenges — and promises — in taking rare disease drugs from research through development, approval, manufacturing and delivery to patients. Rare diseases have small patient populations, which makes developing drugs to treat them inherently more difficult, costly, and risky than those for common medical conditions. The ODTC incentivizes biotechnology and pharmaceutical companies to invest in the development of treatments that are not otherwise economically viable.
In 1983, recognizing rare diseases historically have attracted minimal attention, Congress established the Orphan Drug Act (ODA), creating the tax credit for advancement of rare disease treatments. At the time, only 38 drugs had been approved by the U.S. Food and Drug Administration (FDA) for rare diseases. Now, thanks to this effective and long-standing public health policy, there are more than 650 drugs approved to treat rare diseases. Most importantly, this has led to a significant and consistent decline in the number of annual deaths from rare diseases.
Congress should not be focused on snatching hope from those living with rare diseases; our representatives should instead support and enhance existing rare disease policy because it is working. Of note, the FDA affirmed the value of the ODA and this incentive in an Office of Inspector General (OIG) report issued in September. Rep. Anna Eshoo (D-Calif.) also recently called for the complete restoration of the ODTC after it was reduced from 50 to 25 percent in 2017.
Continue Reading at The Hill.com
Coalition Welcomes New Company Members Healx and Zogenix, Inc.
WASHINGTON, D.C., – December 15, 2021 – The Rare Disease Company Coalition, a unified voice of life science companies committed to discovering, developing and delivering rare disease treatments, today announced the addition of two companies to the Coalition. Following its launch in May of 2021, the Coalition is rounding out its inaugural year boasting a total membership roster of 21 companies.
New members joining the Rare Disease Company Coalition are:
The Coalition strives to inform key policy stakeholders and members of Congress on the unique considerations of life science companies with respect to the development and manufacture of rare disease therapies as well as access and reimbursement following approval. As the federal government continues to consider policy reforms that could impact the ability of companies to innovate and develop groundbreaking treatments, the work of the Coalition and its member companies is essential on behalf of the one in 10 Americans living with rare diseases whose needs often go unmet.
“As we conclude an incredible inaugural year, we welcome two new members to our coalition and remain fiercely dedicated to our work ahead as we continue to facilitate ongoing education and engagement with policymakers about the needs of companies dedicated to the challenging work of developing treatments for rare diseases,” said Betsy Ricketts, Chair of the Rare Disease Company Coalition. “These efforts would not be possible without such an impressive group of life science companies with whom we are grateful to be working. The foundation we have established has us optimistic about the opportunities for continued member growth and greater understanding of rare disease.”
Life science companies that develop treatments for rare disease patients face numerous challenges that include smaller patient populations, variable disease progression or limited understanding of progression, and lack of consensus around meaningful clinical endpoints. As a result, a comparatively higher percentage of operating expenses is dedicated to research and development at these life science companies. In 2020, Rare Disease Company Coalition members invested more than $4.5 billion in research and development, representing on average more than half of their annual operating budgets, with over 225 treatment development programs in process.
This necessary commitment to research and development underscores the importance of supportive policy and regulatory frameworks that can foster a greater understanding of rare diseases and recognizes the distinct considerations associated with the development of and access to rare disease treatments.
To learn more about the Coalition and the need for informed policy and legislative discourse and actions related to rare disease treatment development and commercialization, please visit rarecoalition.com.
About
The Rare Disease Company Coalition represents life science companies committed to discovering, developing and delivering rare disease treatments for the patients we serve. As an education and advocacy-focused coalition of companies, our goal is to inform policymakers of the unique challenges and promises of rare disease drug discovery, development and manufacturing for small population sizes in order for critical innovation to continue. To achieve this goal, we will use our unified voice to advocate for long-term, consistent, equitable and sustainable government policies that enable life science companies to continue to bring hope and provide access to approved treatments to people living with rare diseases. For more information, please visit rarecoalition.com
Media Contact:
Anna Stallmann
media@rarecoalition.com
708-476-1258
WASHINGTON – November 17, 2021 – The Rare Disease Company Coalition, a unified voice of life science companies committed to discovering, developing and delivering rare disease treatments, welcomes the introduction of the bipartisan “Cures 2.0,” legislation by Representatives Diana DeGette (D-CO) and Fred Upton (R-MI) and encourages further engagement with Congressional leaders as it advances through the committee process.
“We applaud the continued leadership by Congresswoman DeGette and Congressman Upton to build on the success of the 21st Century Cures Act, and advance legislation that can better deliver modern treatments and cures to the countless Americans and rare disease patients that await options. As a diverse coalition representing life science companies dedicated to developing and delivering treatments for rare diseases, we look forward to continuing to work with Congressional stakeholders to provide feedback on the unique challenges and opportunities to unleash the promise of our R&D efforts aimed at tackling some of the most complex and devastating rare diseases,” said Betsy Ricketts, Chair of the Rare Disease Company Coalition.
About
The Rare Disease Company Coalition represents life science companies committed to discovering, developing and delivering rare disease treatments for the patients we serve. As an education and advocacy-focused coalition of companies, our goal is to inform policymakers of the unique challenges and promises of rare disease drug discovery, development and manufacturing for small population sizes in order for critical innovation to continue. To achieve this goal, we will use our unified voice to advocate for long-term, consistent, equitable and sustainable government policies that enable life science companies to continue to bring hope and provide access to approved treatments to people living with rare diseases. For more information, please visit rarecoalition.com
Media Contact:
Anna Stallmann
media@rarecoalition.com
708-476-1258
Coalition Calls on Congress to Not Stall Medical Progress for Those Urgently Awaiting Rare Disease Treatment Development
WASHINGTON – November 3, 2021 – The Rare Disease Company Coalition, a unified voice of life science companies committed to discovering, developing and delivering rare disease treatments, today issued the following statement condemning the proposed legislation to amend the Orphan Drug Tax Credit included in the Build Back Better Act, citing the urgent need for continued investment in research and development for the 93 percent of rare diseases that do not have a single treatment available.
“The language released today by the House Budget Committee is disappointing. We have come to the table with solutions and options to address or alleviate potential concerns related to the Orphan Drug Tax Credit,” said Taylor Mason, Executive Director of the Rare Disease Company Coalition. “Unfortunately, the language provided today puts politics over patients. We will continue to fight to ensure continued innovation remains viable for those desperately seeking access to lifesaving treatments, and we hope those in Congress put politics aside, come to the table and do what is right.”
For more information, please find the following resources available:
The 1983 Orphan Drug Act was passed and built upon to help incentivize investment in rare disease research and therapeutic development where there was little research being done and nearly no treatment options for those diagnosed with a rare disease. Over the last several decades, the Act has proven to be a universal success, enabling life science companies to address a growing number of unmet needs for people living with rare diseases that once were untreatable. Since implementation of the Orphan Drug Act in 1983, there have been more than 1,000 FDA approvals for rare disease treatments, with over 25 percent of those approvals occurring in the last three years. The Orphan Drug Tax Credit, already diminished in 2017 under the Tax Cut and Jobs Act from 50 percent to 25 percent, remains a critical program for sustained innovation and investment for innovator companies that exclusively focus on life-changing development programs for treatments for rare diseases.
The Rare Disease Company Coalition was established in 2021 to help advance policies and regulations that enable the cost-effective and timely development and commercialization of rare disease treatments by educating policymakers on the distinct considerations of life science companies operating in the rare disease space. To learn more about the Coalition and its commitment to rare disease treatment development and commercialization, please visit rarecoalition.com.
About
The Rare Disease Company Coalition represents life science companies committed to discovering, developing and delivering rare disease treatments for the patients we serve. As an education and advocacy-focused coalition of companies, our goal is to inform policymakers of the unique challenges and promises of rare disease drug discovery, development and manufacturing for small population sizes in order for critical innovation to continue. To achieve this goal, we will use our unified voice to advocate for long-term, consistent, equitable and sustainable government policies that enable life science companies to continue to bring hope and provide access to approved treatments to people living with rare diseases. For more information, please visit rarecoalition.com
Media Contact:
Anna Stallmann
media@rarecoalition.com
708-476-1258






